Key Points
The study describes a potential novel treatment of fetal alloimmune thrombocytopenia by dissecting the effector activities of an epitope-specific IgG antibody.
Neither the in vivo transplacental transport nor the inhibiting properties of the blocking antibody are impaired by the N-glycan modification.
Abstract
Fetal/neonatal alloimmune thrombocytopenia (FNAIT) is often caused by maternal alloantibodies against the human platelet antigen (HPA)–1a, which opsonizes fetal platelets (PLTs). Subsequent PLT destruction is mediated via the Fc part of the alloantibodies. The monoclonal antibody (mAb) SZ21 binds to the HPA-1a epitope and inhibits the binding of maternal alloantibodies. However, it also promotes complement activation and phagocytosis. Deglycosylation of antibodies abrogates the Fc-related effector functions. We modified the N-glycan of SZ21 by endoglycosidase F. The in vivo transplacental transport of N-glycan–modified (NGM)-SZ21 was not impaired. When injected into pregnant mice, both native-SZ21 and NGM-SZ21 were transported equally into fetal circulation (8.9% vs 8.7%, respectively, P = .58). Neither the binding properties of NGM-SZ21 to HPA-1a in surface plasmon resonance, nor the inhibition of anti–HPA-1a–induced PLT phagocytosis, were affected by N-glycan modification. NGM-SZ21 prevented PLT destruction induced by maternal anti–HPA-1a antibodies in vivo in a mouse model (PLT clearance after 5 hours; 18% vs 62%, in the presence or absence of NGM-SZ21, respectively, P = .013). Deglycosylation of SZ21 abrogates Fc-effector functions without interfering with placental transport or the ability to block anti–HPA-1a binding. Humanized, deglycosylated anti–HPA-1a mAbs may represent a novel treatment strategy to prevent anti–HPA-1a–mediated PLT destruction in FNAIT.
Introduction
Fetal/neonatal alloimmune thrombocytopenia (FNAIT) results from maternal immunization against fetal-specific human platelet antigens (HPAs).1-3 Analogous to Rhesus D incompatibility, maternal antibodies to alloantigens (blood groups) on fetal platelets can result in the destruction of fetal platelets after transplacental transport from the maternal to the fetal circulation. Alloantibodies against the epitope HPA-1a on glycoprotein (GP) IIb-IIIa are responsible for most of the severe cases of FNAIT.4-7 The incidence of HPA-1a–mediated FNAIT in the Caucasian population is about 1 in 1500 live births, based on a large population study,7 with no prophylactic measures to prevent maternal immunization.7 The most devastating risk of FNAIT is intracranial hemorrhage, which may lead to death or persistent neurological sequel in 10% of the clinically symptomatic cases.4,8 After delivery, FNAIT can be treated by platelet transfusion.9,10 However, in almost 50% of affected cases, intracranial hemorrhage occurs before delivery, sometimes as early as in the 20th week of gestation.8,11,12 This makes antenatal treatment essential to avoid deleterious consequences.5 Ideally, treatment should be initiated from about the 20th week of gestation, as from then on the placenta transports maternal IgG to the fetus and fetal platelets already express the HPAs.13-15
Currently, antenatal treatment options include intrauterine platelet transfusion to the fetus or treatment of the pregnant mother with intravenous immunoglobulin with or without additional steroids.1,3 All 3 treatment options have limitations. Intrauterine platelet transfusion is associated with the risk of severe procedure-related complications causing iatrogenic fetal death.16 High-dose steroids for 12 to 20 weeks during gestation increase the risk for gestational diabetes and put the mother at an increased risk for infection, and little is known about the long-term effects of immunomodulation of the mother during pregnancy. In addition, these treatments have limited efficacy. About 20% of the newborns remain severely thrombocytopenic despite treatment of the mother with intravenous immunoglobulin and steroids.12,17
As fetal platelet destruction is initiated after the binding of maternal alloantibodies to the fetal platelet surface, an attractive treatment option would be to block the binding of these maternal alloantibodies to the respective alloantigens on fetal platelets. Recently, we demonstrated the protective effect of Ag-binding fragments (F(ab)′2) of the monoclonal antibody (mAb) SZ21 on platelet clearance induced by maternal anti–HPA-1a alloantibodies.18 This mAb binds to the HPA-1a epitope and competes with the human alloantibodies. As the mother lacks the antigen to which it binds, one could safely inject the mAb SZ21 into the mother, taking advantage of the maternofetal transport of antibodies. However, this concept has 2 major practical obstacles. Monoclonal antibodies with an intact Fc moiety are as effective as maternal alloantibodies in inducing platelet destruction in vivo via Fc receptors, while F(ab)′2 fragments are not efficiently transported across the placenta to the fetus.
IgG is transported from the maternal circulation to the fetus by binding to the neonatal Fc receptor of FcRn that is expressed in the placental villous syncytiotrophoblast.19,20 FcRn-mediated IgG transport does not require carbohydrate moieties on the Fc portion of the antibody for binding or transplacental transport.15,21 Thus, removal of the N-glycan should not affect placental transport. In contrast, deglycosylation of the N-glycan attached to Asn297 inhibits recognition by Fc receptors on macrophages (FcRI, FcRIIa, FcRIIIa) as well as its ability to activate complement factor C1q.22,23
In this study, we demonstrate that deglycosylation of the anti–HPA-1a mAb SZ21 neither affects its efficient transplacental transport nor its ability to bind with high affinity to the HPA-1a epitope on platelets, thereby blocking the binding of maternal HPA-1a alloantibodies and subsequent destruction of HPA-1a platelets in vivo. These studies may indicate a new approach for a minimally invasive treatment strategy for the prevention of fetal platelet destruction by maternal anti–HPA-1a alloantibodies.
Material and methods
Antibodies
mAb SZ21, which binds the epitope HPA-1a on GPIIIa, was obtained from Beckman Coulter (Krefeld, Germany). Isotype-matched mouse IgG1 was obtained from Beckman Coulter. mAb AP2, which recognizes a complex-dependent epitope on GPIIb-IIIa but does not interfere with the HPA-1a epitope, was kindly provided by Dr Robert R. Montgomery (Blood Research Institute, Milwaukee, WI). The deglycosylated variants of these 2 antibodies are named N-glycan–modified (NGM)-SZ21 and NGM-AP2, respectively. Human anti–HPA-1a alloantibodies were obtained from sera of women who developed these antibodies during pregnancies that were complicated with severe FNAIT and were purified by Melon Kit methodology (Thermo Fisher Scientific, Bonn, Germany). The animal experiments were approved by the local animal authorities in Hesse, Germany, and Milwaukee, Wisconsin.
N-glycan modification of mAbs
To deglycosylate the mAbs, the N-linked glycan attached to Asn297 of the IgG heavy chain was enzymatically removed under native conditions using endo F (Native Protein Deglycosylation Kit; Sigma-Aldrich, Munich, Germany). In brief, 200 µg SZ21 or isomatched mAb (AP2) were incubated with 2 µL of endo F in phosphate-buffered saline at 37°C for 2 hours and then purified using the Melon IgG purification kit (Thermo Fisher Scientific).
Degradation of antibodies by endo F treatment was evaluated by Coomassie blue–stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Efficiency of antibody deglycosylation was investigated using lectin blotting. A total of 1 µg SZ21 or NGM-SZ21 was resolved by 10% to 20% precast gradient SDS-PAGE (Bio-Rad, Munich, Germany) under reducing conditions and transferred to polyvinylidene fluoride membrane. Biotinylated Lens culinaris agglutinin (LCA) (Sigma-Aldrich) was added to a final concentration of 50 µg/mL for 45 minutes at room temperature (RT) and the membrane was washed 10 times (0.05% Tween/tris(hydroxymethyl)aminomethane–buffered saline). Subsequently, peroxidase-conjugated streptavidin (Sigma-Aldrich) was added in a final concentration of 1 µg/mL for 30 minutes at RT and bound LCA was visualized by enhanced chemiluminescence detection kit (GE Healthcare, Munich, Germany).
To further analyze the specificity of antibody deglycosylation, SZ21 and NGM-SZ21 were separated on SDS-PAGE as described above. Gel matrix containing IgG heavy chain was extracted, digested, and analyzed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Voyager-DE Biospectrometry workstation; Applied Biosystems, Foster City, CA).
Assessment of transplacental maternofetal transport of NGM-SZ21
For maternal antibody transfer, age-matched pregnant female BALB/c mice at 17 days of gestation were injected IV with a total of 40 µg SZ21, NGM-SZ21, or isotype-matched mouse IgG 1 (Beckman Coulter). After delivery, that is, 3 to 4 days after antibody injection into the pregnant mother, blood was collected from the 1- to 8-hours-old pups by carotid bleeding. After pooling blood samples from all pups of each pregnancy, sera were obtained to assess the amount of free anti–HPA-1a alloantibodies in the neonatal mouse blood using GPIIIa surface plasmon resonance (SPR) as described in “Antibody-binding characterization using SPR.” This experiment was done in triplicate.
Antibody-binding characterization using SPR
The binding kinetic of NGM-SZ21 was analyzed by SPR technology using ProteOn XPR36 (Bio-Rad) as described in Bakchoul et al.24 In brief, GPIIb-IIIa from human platelets was isolated using affinity chromatography and immobilized onto flow cells of a GLM sensor chip (25 µg in 250 µL ProteOn acetate buffer, flow rate 30 µL/minute until saturation). Bovine serum albumin was immobilized onto the control flow cell.
To quantify the mAbs transported across the placenta into the fetal circulation, we used both variants of SZ21 (native and NGM) as standards, as previously described in Bakchoul et al.25 The mAbs were initially diluted in reaction buffer to a concentration of 20 ng/mL. A standard curve was constructed with a nonlinear 4-parameter logistic regression using Prism version 5.0 (GraphPad, La Jolla, CA) by plotting the response units (RUs, means of duplicates) of fetal or maternal blood samples against 6 dilutions of the reference sample. The RU values were then read against this standard curve.
To determine the equilibrium dissociation constant (KD) of the mAbs, 6 different concentrations with twofold dilution of the maximum concentration 10 nM (1500, 750, 375, 188, 94, and 47 ng/mL) of SZ21 or NGM-SZ21 were injected over the coated sensor chips at a flow rate of 30 µL/minute. Antibody binding was evaluated during an association phase (antibody injection, 0-350 seconds), which was followed by a dissociation phase (injection of buffer only, 350-700 seconds). The association phase, where the antibody is flowed across the coated sensor chips and binding is measured, allows the determination of the rate of formation of the antibody-antigen complex over the time that is reflected by an increase in the RUs. The kinetic of the increase in RU determines the association constant (Ka). In the dissociation phase, the antibody is removed from the flow (concentration in buffer is 0) and the rate of complex dissociation follows exponential decay kinetics. This kinetic determines the dissociation constant (Kd). Data were double referenced by subtraction of the control flow cell and data from interspots, as recommended by the manufacturers. Antibody affinity to GPIIb-IIIa and rate constants of the antibody-antigen interactions were determined by global analysis using a simple 1:1 Langmuir binding model provided by the ProteOn X36 software (Bio-Rad). Further details are explained in “Results.”
Antibody-mediated platelet phagocytosis assay
To test the ability of NGM-SZ21 to prevent maternal anti–HPA-1a–induced platelet phagocytosis in vitro, a platelet phagocytosis assay was performed. Monocytes were isolated from peripheral blood samples of healthy donors (HPA-1ab, blood group O) by anti-CD14 MicroBeads using autoMACS technology according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Isolated monocytes were adjusted to 1 × 104/µL in 10% fetal calf serum–RPMI (PAA Laboratories, Pasching, Austria).
Platelets were isolated by centrifugation from 10 mL citrated whole blood of the monocyte donor (72 g for 25 minutes at RT). One hundred microliters platelet rich plasma (1 × 105/µL) were labeled with 2 µL of 1 mM CMFDA (an intracellular dye, CellTracker green CMFDA; Molecular Probes Invitrogen, Karlsruhe, Germany) for 45 minutes at RT. Excessive CMFDA was then removed and platelet concentration was adjusted to1 × 103/µL.
Aliquots of 100 µL labeled platelets were preincubated with NGM-SZ21 or NGM-AP2 (5, 15, 30, and 60 µg/mL) for 15 minutes at RT. Fifty microliters IgG fraction of human sera containing anti–HPA-1a antibodies were added to the suspension for a further 30 minutes. Opsonized platelets were then incubated with monocytes (ratio 1:10) for 2 hours at 37°C. Thereafter, monocytes were labeled with 5 µL phycoerythrin (PE)–labeled anti-CD14 mAb (Becton, Dickinson GmbH, Heidelberg, Germany) and analyzed by flow cytometry (BD FACSCanto; BD Biosciences, San Jose, CA). Monocytes (PE-positive events) were gated and the percentage of fluorescein isothiocyanate (FITC)–positive monocytes, that is, those that ingested labeled platelets, was identified as the phagocytic activity. Human anti–HPA-1a alloantibodies were tested in triplicate with monocytes and platelets of 3 different donors.
Analysis of maternal alloantibody-mediated HPA-1a platelet destruction in vivo
The survival of human platelets was investigated in the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse model for alloimmune thrombocytopenia as described in Bakchoul et al18 and Boylan et al.26 Thirty micrograms NGM-SZ21 or NGM-AP2 were added to 200 µL resting platelets (2 × 109/µL) from healthy donors in autologous plasma before injection into the retroorbital plexus of NOD/SCID mice (The Jackson Laboratory, Bar Harbor, ME). After 30 minutes, a blood sample was collected to define the baseline of circulating human platelets (100%) and 800 µg of the human anti–HPA-1a IgG fraction were intraperitoneally injected. Survival of human platelets in the mouse circulation was estimated by analyzing blood samples obtained at 60, 180, and 300 minutes after antibody injection by flow cytometry using FITC-labeled Gi5 and PE-labeled MWreg30 (Becton, Dickinson GmbH) specific for human and mouse GPIIb-IIIa, respectively.
Statistical analyses
Statistical analyses were performed using Prism version 5.0 (GraphPad). Comparisons between 2 groups were calculated using Student t test, and between 3 and more groups using 1-way analysis of variance test and Mann-Whitney U test. P values < .05 were considered statistically significant.
Results
N-glycan modification of mAb SZ21 using endo F
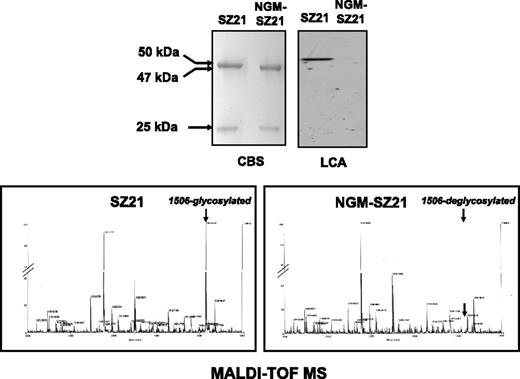
To modify the N-glycan of the mAbs, deglycosylation was performed under native conditions using endo F, and the degradation of antibodies was evaluated by Coomassie blue–stained SDS-PAGE. Untreated SZ21 showed 2 bands in SDS-PAGE under reducing conditions with apparent molecular weights of 50 kilodalton (kDa) (heavy chain) and 25 kDa (light chain) (Figure 1). Treatment with endo F resulted in a reduction of ∼3 kDa of the apparent weight of the heavy chain, but not of the light chain of NGM-SZ21. No additional bands were detected.
N-glycan modification of the anti–HPA-1a mAb SZ21. Coomassie blue staining (CBS) was used to analyze the general structure modifications of SZ21 after its treatment with endo F. Two bands with apparent molecular weights of <50 kDa (∼47 kDa) and 25 kDa representing the heavy and light chains of mAb SZ21 and NGM-SZ21 were visible under reducing conditions (upper left panel). N-glycan was then detected using LCA (upper right panel). A strong signal was solely detected at a molecular weight of ∼50 kDa of the lane loaded with SZ21 (lane 1). No signal was detected by testing endo F–treated NGM-SZ21 (lane 2), indicating the removal of the N-glycan. The specificity of deglycosylation was analyzed by MALDI-TOF mass spectroscopy (MS). As shown in the lower panels, the only difference between the heavy chain of mAb SZ21 (left panel) and the heavy chain of NGM-SZ21 (right panel) was in fragment 1506 corresponding to the N-glycan that is linked to the asparagine at position 297. No additional modifications were observed in the heavy chain of the NGM-SZ21.
N-glycan modification of the anti–HPA-1a mAb SZ21. Coomassie blue staining (CBS) was used to analyze the general structure modifications of SZ21 after its treatment with endo F. Two bands with apparent molecular weights of <50 kDa (∼47 kDa) and 25 kDa representing the heavy and light chains of mAb SZ21 and NGM-SZ21 were visible under reducing conditions (upper left panel). N-glycan was then detected using LCA (upper right panel). A strong signal was solely detected at a molecular weight of ∼50 kDa of the lane loaded with SZ21 (lane 1). No signal was detected by testing endo F–treated NGM-SZ21 (lane 2), indicating the removal of the N-glycan. The specificity of deglycosylation was analyzed by MALDI-TOF mass spectroscopy (MS). As shown in the lower panels, the only difference between the heavy chain of mAb SZ21 (left panel) and the heavy chain of NGM-SZ21 (right panel) was in fragment 1506 corresponding to the N-glycan that is linked to the asparagine at position 297. No additional modifications were observed in the heavy chain of the NGM-SZ21.
The efficiency of antibody deglycosylation was investigated using lectin blotting. By lectin blotting, LCA bound to the heavy chain of untreated SZ21 but not to that of NGM-SZ21 (Figure 1), indicating efficient removal of the IgG glycan. Removal of the N-linked glycan at Asn297 residue under native conditions was also documented by MALDI-TOF mass spectroscopy. The mass chromatogram (Figure 1) revealed that the major glycan present in SZ21 (fragment 1506) was absent in the heavy chain of NGM-SZ21. No additional cleavage was observed in the heavy chain of NGM-SZ21. These results indicate that endo F treatment only removed the N-glycan attached to Asn297 of the antibody heavy chain without altering the protein structures.
N-glycan modification does not impair the transplacental transport of SZ21
To investigate transplacental transport, 3 pregnant BALB/c mice were IV injected with 40 µg SZ21, NGM-SZ21, or isotype-matched control IgG antibody at gestation day 17. Maternal blood samples (100 µL) were collected from pregnant mice 5 minutes after injection and within 8 hours after delivery. Blood samples from newborn pups were collected within 8 hours after birth. Because of the small collected volume (10-25 µL), neonatal blood samples of pups of 1 pregnancy were pooled together and the respective IgG fractions were isolated from 50 µL pooled sera for SPR analysis.
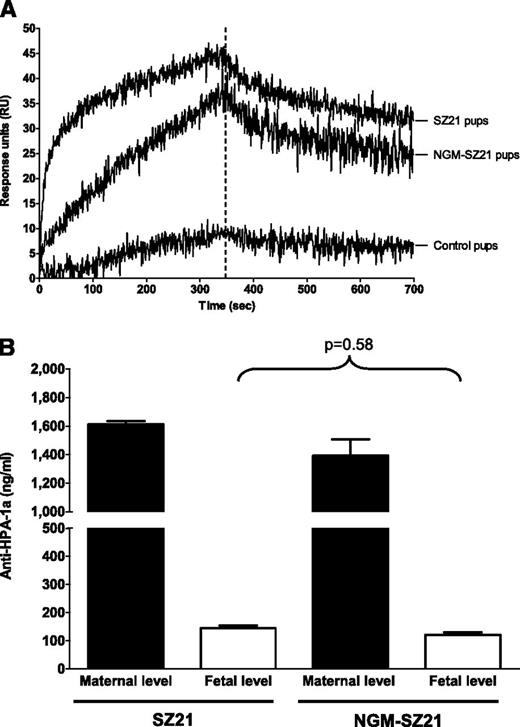
By SPR, we found SZ21 and NGM-SZ21 in the blood of the pups (Figure 2A). As these antibodies had been IV injected into pregnant mother mice, they must have been transported via the placenta to the pups. At the end of the association phase (B350), 44 RU ± 4 standard deviation and 38 RU ± 5, respectively, and at the end of the dissociation phase (B700), 32 RU ± 3 and 25 RU ± 4, respectively, were found for SZ21 and NGM-SZ21. These data demonstrate that the deglycosylated antibody is transported with the same efficacy through the placenta as is the glycosylated antibody. No relevant binding was detected with the IgG fraction of the control pups (Figure 2A).
The impact of the N-glycan on the in vivo transport of monoclonal antibodies through the placenta. To investigate the effect of N-glycan on IgG transport, a total of 40 µg SZ21, NGM-SZ21, or isotype-matched (nonplatelet-binding) IgG were injected in age-matched pregnant female BALB/c mice at day 17 of gestation. Blood samples from newborn pups were collected within 8 hours after birth. (A) Binding of transported antibodies is shown. In SPR, similar antibody binding to GPIIb-IIIa from HPA-1aa platelets was detected in the blood of pups whose mothers were injected with either SZ21 or NGM-SZ21. No relevant antibody binding was detected using the blood of the control pups. (B) Quantification of circulating antibodies is shown. An equal amount of SZ21 and NGM-SZ21 (8.9% and 8.7%, respectively, P = .58) was transported from maternal (black bars) into neonatal (white bars) circulation at the end of the pregnancy. This figure shows data from 3 different experiments.
The impact of the N-glycan on the in vivo transport of monoclonal antibodies through the placenta. To investigate the effect of N-glycan on IgG transport, a total of 40 µg SZ21, NGM-SZ21, or isotype-matched (nonplatelet-binding) IgG were injected in age-matched pregnant female BALB/c mice at day 17 of gestation. Blood samples from newborn pups were collected within 8 hours after birth. (A) Binding of transported antibodies is shown. In SPR, similar antibody binding to GPIIb-IIIa from HPA-1aa platelets was detected in the blood of pups whose mothers were injected with either SZ21 or NGM-SZ21. No relevant antibody binding was detected using the blood of the control pups. (B) Quantification of circulating antibodies is shown. An equal amount of SZ21 and NGM-SZ21 (8.9% and 8.7%, respectively, P = .58) was transported from maternal (black bars) into neonatal (white bars) circulation at the end of the pregnancy. This figure shows data from 3 different experiments.
Quantification of circulating SZ21 and NGM-SZ21 in the maternal and neonatal circulation was performed against the corresponding standard curves. In maternal IgG fractions, concentrations of 2190 ± 15 ng/mL and 1888 ± 123 ng/mL of SZ21 and NGM-SZ21, respectively, were measured 5 minutes after antibody injection, of which 1613 ± 21 ng/mL (71%) and 1393 ± 114 ng/mL (72%) were still detectable 8 hours after delivery (gestation day 21 ± 1 day) (Figure 2B). Eight hours after delivery, concentrations of 145 ± 18 ng/mL SZ21 and 115 ± 16 ng/mL NGM-SZ21 were determined in the pups, indicating that approximately 9% of SZ21 and NGM-SZ21 (P = .58) had been transported from the maternal into the fetal circulation (Figure 2B). To compare antibody quantification by SPR with solid phase enzyme-linked immunosorbent assay, purified GPIIb-IIIa–complex was coated on the microtiter plate and antibody binding was measured by the use of enzyme-labeled secondary antibodies. Comparable standard curves between enzyme-linked immunosorbent assay and SPR were obtained (see supplemental Figure 1 on the Blood website).
NGM-SZ21 displays binding properties similar to unmodified SZ21
To investigate the impact of N-glycan removal on the binding affinity of SZ21 for the HPA-1 epitope on GPIIb-IIIa, we compared untreated mAb SZ21 and NGM-SZ21 using SPR. SZ21 and NGM-SZ21 were injected over the coated sensor chips in 6 different concentrations (range: 1500-47 ng/mL). Antibody binding was analyzed during an association phase and a dissociation phase. For SZ21, a Ka of 4.7 × 10−5 M and a Kd of 5.5 × 10−4 M were observed, giving an estimated KD of 1.17 × 10−9 M, which compares to NGM-SZ21, for which a Ka of 8.6 × 10−4 M and a Kd of 3.76 × 10−4 M were determined, resulting in an estimated KD of 4.36 × 10−9 M. When analyzed using GPIIb-IIIa from HPA-1bb homozygous platelets, low-affinity binding was documented. Both antibodies showed similar high dissociation rates, and consequently higher Kd values, 8.36 × 10−8 M and 1.78 × 10−8 M, respectively. Taken together, these data confirm that N-glycan removal did not impair the binding affinity of SZ21 to the HPA-1a epitope.
NGM-SZ21 inhibits platelet phagocytosis induced by maternal HPA-1a alloantibodies
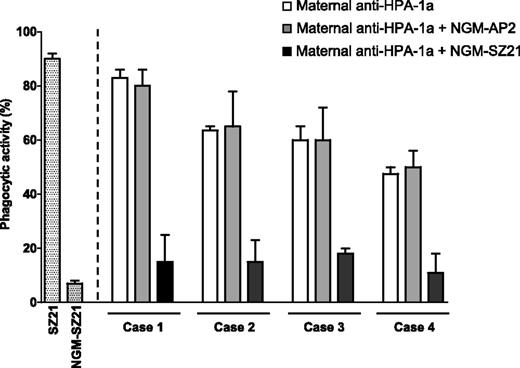
To test the impact of N-glycan removal on the phagocytic activity of SZ21, a platelet phagocytosis assay was used. While SZ21 induced a high platelet phagocytic activity, opsonization with NGM-SZ21 did not cause significant phagocytosis (Figure 3).
Ability of NGM-SZ21 to prevent anti–HPA-1a–mediated phagocytosis of HPA-1a platelets. The effect of antibody deglycosylation on the phagocytic activity was investigated by opsonizing CMFDA (FITC)–labeled platelets with SZ21 or NGM-SZ21 before adding the monocytes. Monocytes were then gated and the percentage of FITC-positive monocytes, that is, those that ingested labeled platelets, was identified as the phagocytic activity (%). Note that SZ21 but not NGM-SZ21 was capable of inducing platelet phagocytosis (white and gray bars, respectively). To assess the protection property, labeled platelets were incubated with NGM-SZ21 (black bars) or with NGM-AP2 as an isotype-matched control (gray bars) before adding maternal anti–HPA-1a IgG. Then monocytes were added and phagocytosis of the opsonized platelets was assessed and expressed as phagocytic activity. White bars represent the native phagocytic activity of the maternal anti–HPA-1a antibodies. Gray bars show that the control antibody NGM-AP2 did not inhibit phagocytosis, while the black bars show inhibition of the phagocytic activity by NGM-SZ21 (median of inhibition: 47% vs 0%, respectively, P = .008).
Ability of NGM-SZ21 to prevent anti–HPA-1a–mediated phagocytosis of HPA-1a platelets. The effect of antibody deglycosylation on the phagocytic activity was investigated by opsonizing CMFDA (FITC)–labeled platelets with SZ21 or NGM-SZ21 before adding the monocytes. Monocytes were then gated and the percentage of FITC-positive monocytes, that is, those that ingested labeled platelets, was identified as the phagocytic activity (%). Note that SZ21 but not NGM-SZ21 was capable of inducing platelet phagocytosis (white and gray bars, respectively). To assess the protection property, labeled platelets were incubated with NGM-SZ21 (black bars) or with NGM-AP2 as an isotype-matched control (gray bars) before adding maternal anti–HPA-1a IgG. Then monocytes were added and phagocytosis of the opsonized platelets was assessed and expressed as phagocytic activity. White bars represent the native phagocytic activity of the maternal anti–HPA-1a antibodies. Gray bars show that the control antibody NGM-AP2 did not inhibit phagocytosis, while the black bars show inhibition of the phagocytic activity by NGM-SZ21 (median of inhibition: 47% vs 0%, respectively, P = .008).
To investigate the ability of NGM-SZ21 to inhibit the phagocytosis of platelets induced by maternal anti–HPA-1a alloantibodies, HPA-1a–positive platelets were treated with NGM-SZ21 or NGM-AP2 before adding human anti–HPA-1a antibodies (n = 4). A marked reduction of anti–HPA-1a–mediated platelet phagocytosis was observed after preincubation with NGM-SZ21 but not with NGM-AP2 (Figure 3).
NGM-SZ21 prevents maternal HPA-1a alloantibody-mediated platelet clearance in vivo
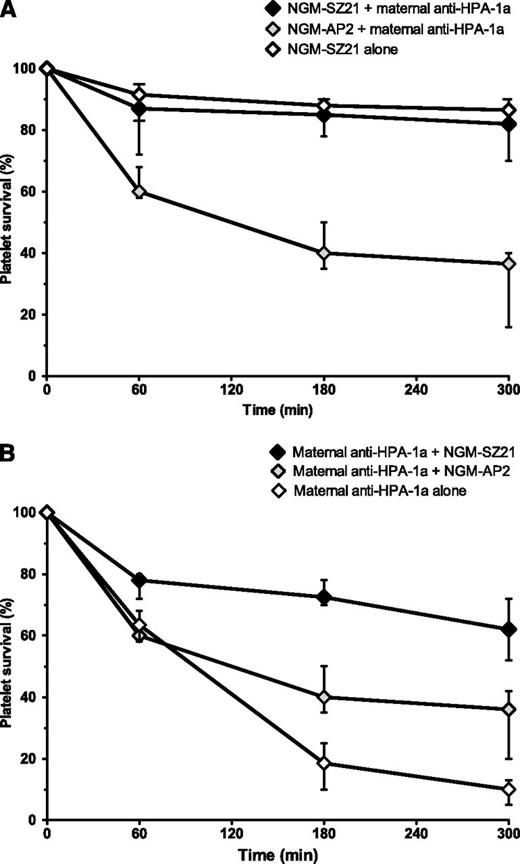
The ability of NGM-SZ21 to prevent anti–HPA-1a–mediated clearance of human platelets in vivo was investigated by injecting NGM-SZ21 (HPA-1a epitope specific) before the injection with anti–HPA-1a alloantibodies (n = 4). NGM-AP2 (HPA-1a epitope nonspecific) was used as the control. Consistent with the results of the in vitro experiments described above, platelet clearance was significantly inhibited by the administration of NGM-SZ21 but not by NGM-AP2 (median of human platelet clearance (CL5h): 18%, range: 10%-30% vs 62%, range: 60-84, P = .013) (Figure 4A).
NGM-SZ21 prevents anti–HPA-1a antibody-mediated platelet destruction in a NOD/SCID mouse model of alloimmune thrombocytopenia. Resting human platelets (HPA-1ab) were injected retroorbitally into NOD/SCID mice and the survival of platelets was analyzed. Results are shown as a median and range of experiments that were performed in duplicate with anti–HPA-1a antibodies from 4 FNAIT cases. (A) Epitope blockade: When NGM-SZ21 was injected before the maternal anti–HPA-1a antibodies, the elimination of platelets was largely inhibited (black symbols), while the control antibody NGM-AP2 did not prevent rapid elimination by human anti–HPA-1a antibodies (gray symbols). NGM-SZ21 alone did not induce relevant platelet destruction (white symbols). (B) Antibody competing: To simulate the situation of advanced pregnancy, maternal anti–HPA-1a antibodies were injected 30 minutes before giving NGM-SZ21 or NGM-AP2. The injection of maternal anti–HPA-1a alone resulted in a rapid destruction of circulating human HPA-1a platelets (white symbols). Note that NGM-SZ21 (black symbols) but not NGM-AP2 (gray symbols) was able to ameliorate platelet destruction induced by the circulation of maternal anti–HPA-1a antibodies.
NGM-SZ21 prevents anti–HPA-1a antibody-mediated platelet destruction in a NOD/SCID mouse model of alloimmune thrombocytopenia. Resting human platelets (HPA-1ab) were injected retroorbitally into NOD/SCID mice and the survival of platelets was analyzed. Results are shown as a median and range of experiments that were performed in duplicate with anti–HPA-1a antibodies from 4 FNAIT cases. (A) Epitope blockade: When NGM-SZ21 was injected before the maternal anti–HPA-1a antibodies, the elimination of platelets was largely inhibited (black symbols), while the control antibody NGM-AP2 did not prevent rapid elimination by human anti–HPA-1a antibodies (gray symbols). NGM-SZ21 alone did not induce relevant platelet destruction (white symbols). (B) Antibody competing: To simulate the situation of advanced pregnancy, maternal anti–HPA-1a antibodies were injected 30 minutes before giving NGM-SZ21 or NGM-AP2. The injection of maternal anti–HPA-1a alone resulted in a rapid destruction of circulating human HPA-1a platelets (white symbols). Note that NGM-SZ21 (black symbols) but not NGM-AP2 (gray symbols) was able to ameliorate platelet destruction induced by the circulation of maternal anti–HPA-1a antibodies.
To simulate the situation of advanced pregnancy during which maternal anti–HPA-1a alloantibodies are already present in the fetal circulation, we injected maternal anti–HPA-1a antibodies into mice 30 minutes before giving NGM-SZ21 or NGM-AP2. NGM-SZ21 but not NGM-AP2 was able to ameliorate platelet destruction induced by the circulation of maternal anti–HPA-1a antibodies (median CL5: 38%, range: 28%-48% vs 64%, range: 58%-80%, P = .023) (Figure 4B).
Taken together, these data suggest that NGM-SZ21 prevents anti–HPA-1a antibody-mediated platelet destruction by competing with the binding of maternal alloantibodies, even when injected at a time point during which the maternal alloantibodies were already present in the circulation.
Discussion
This study provides the basis for a novel strategy to prevent fetal platelet destruction in FNAIT. We show that an engineered HPA-1a–specific mAb, deglycosylated at amino acid Asn297 of its heavy chain, is transported through the placenta as efficiently as is its native, fully glycosylated counterpart. The deglycosylated antibody binds via its Fab domain to the HPA-1a epitope on GPIIb-IIIa. However, it lacks key effector functions that promote undesirable Fc-receptor–dependent phagocytosis and complement activation and effectively blocks platelet phagocytosis and destruction by native human anti–HPA-1a alloantibodies in vitro as well as in vivo. As the human anti–HPA-1a alloantibodies were obtained from women in whom these antibodies induced severe FNAIT during pregnancy, it is very likely that the engineered mAb will also be protective in human FNAIT.
As a proof of principle, we took advantage of the well-characterized mAb SZ21, which specifically binds to the HPA-1a epitope and is well known to block the binding of human anti–HPA-1a antibodies.18,27 We enzymatically removed the N-glycan attached to Asn297 of the antibody heavy chain. This enzymatic digestion was highly effective and specific, as shown by mass spectrometry, and seemed not to alter the protein structure of the antibody, as shown by the lack of protein degradation products. And, most important, it preserved the binding specificity and affinity of the antibody to its antigen, as shown by SPR.
In our in vivo mouse model, the transplacental transport of the deglycosylated antibody NGM-SZ21 was as effective as the transplacental transport of its native form SZ21. In the pup circulation, we recovered about 9% of the concentrations determined in the maternal blood of both forms of the antibody (NGM-SZ21 and native SZ21), which is in accordance with the relative levels of other epitope-specific antibodies recovered in newborns in humans as well as in mice.28,29 The amount of blood we could obtain from the newborn mice was very limited. We therefore had to pool the blood of several of the newborn pups from 1 pregnancy for analysis. Theoretically, it would be possible that the amount of antibody transferred from the maternal to the fetal circulation varied across pups. Due to this limitation, and due to the small number of animals used in our study, we can only assume that the antibodies had been transported to all pups in similar concentrations.
Most important, NGM-SZ21 did prevent platelet phagocytosis induced by different maternal anti–HPA-1a alloantibodies in vitro and also protected against the destruction of HPA-1a–positive platelets by human anti–HPA-1a alloantibodies in vivo in a NOD/SCID mouse model.18,26 Anti–HPA-1a antibody-mediated platelet destruction was also inhibited by NGM-SZ21 when we injected the human anti–HPA-1a alloantibodies before the administration of NGM-SZ21 to better simulate the situation of FNAIT in pregnancy. These results indicate that NGM-SZ21 is capable of preventing anti–HPA-1a–mediated platelet destruction in vivo even when maternal anti–HPA-1a alloantibodies are already present in the fetal circulation. Likely this is mediated by competing with maternal HPA-1a alloantibody binding to fetal platelets or even by displacing the maternal alloantibodies, as previously described in Bakchoul et al18 and Boylan et al26 for the F(ab)′2 fragment of SZ21.
Unfortunately, no mouse model for fetomaternal incompatibility in human platelet alloantigens currently exists. Due to this limitation, we cannot directly assess the capability of deglycosylated epitope-specific monoclonal antibodies to protect fetal mice from platelet destruction by maternal alloantibodies. The existing mouse model of fetomaternal platelet incompatibility employs GPIIb-IIIa knockout mice. These mice develop a broad isoimmune response to multiple epitopes on the large GPIIb-IIIa complex when immunized with platelets of wild-type mice.28 In this model, multiple antibodies with different binding sites on GPIIb-IIIa are transported to the pups. Their binding cannot be blocked by 1 epitope-specific mAb. The development of a novel mouse model for fetomaternal incompatibility using humanized mice carrying the correct human major histocompatibility complex class II that can be challenged by human β3 integrin with HPA-1a and HPA-1b would help in overcoming this limitation.
Due to anatomical and functional differences between human and murine placentas,30-32 the final proof of our concept can only be provided by a clinical study in humans. This will require a humanized form of the mAb SZ21 (or another HPA-1a–specific antibody), a technology well established for other therapeutic antibodies.33,34 For human use, however, there are important issues that cannot be assessed in our study and need to be taken into consideration. It is known that the β-subunit of the αVβ3 integrin on endothelium and placenta bears the HPA-1a epitope(s). This fact may create some problems for the application of the engineered humanized mAb SZ21. First, it is not clear whether endothelial cells expressing the HPA-1a epitope can mop up the antibody. Because the αVβ3 complex on endothelial cells is expressed on the basal, rather than apical, cell surface,35 it is unlikely that humanized mAb SZ21 would be absorbed by maternal endothelial cells. Second, this antibody may react with the fetally derived trophoblasts, which are most probably heterozygous for the HPA-1 polymorphism. However, maternal anti–HPA-1a alloantibodies get past them quite well on their way into the fetal circulation and induce platelet destruction in FNAIT. Therefore, this problem likely can be overcome by adjusting the dose of SZ21 to ensure the delivery of a therapeutically effective dose.
The general concept of “dissociating” the effector activities of an IgG antibody from its transplacental transport potentially may allow the development of preventive treatments of other antibody-mediated fetomaternal incompatibilities and may even provide some perspectives for treating other fetal disorders resulting from transplacental antibody transfer.
In conclusion, removal of the N-glycan of amino acid Asn297 of the heavy chain of a mAb specific for the HPA-1a epitope on GPIIb-IIIa modifies the effector functions of the antibody but still allows its efficient maternofetal transport. This modified antibody prevents in vivo destruction of HPA-1a–positive platelets by maternal anti–HPA-1a alloantibodies. This provides the basis to develop a new approach to prevent severe thrombocytopenia in FNAIT.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The excellent technical support of Annika Krautwurst and Astrid Giptner is gratefully acknowledged.
This work was supported by a research grant from Justus Liebig University Giessen (T.B.), a grant from the Excellence Cluster Cardio-Pulmonary System (S.S.), a grant of the Bundesministerium für Bildung und Forschung, Zentrum für Innovations-kompetenz Humorale Immunantwort in der Kardiovaskularen Erkrankungen Förderkennzeichen BMBF FKZ 03Z2CN12 (A.G.), and a National Institutes of Health, National Heart, Lung, and Blood Institute grant (HL44612) (P.J.N.). This work is a part of the doctoral thesis of H.H.
Authorship
Contribution: T.B. designed and performed the experiments, analyzed the data, and wrote the paper; A.G. analyzed the data, provided constructive criticism, and wrote the paper; A.K. and H.H. performed in vitro and in vivo experiments and collected and analyzed the data; U.J.S., H.R., and G.B. provided novel reagents and important suggestions; and P.J.N. and S.S. designed and supervised the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sentot Santoso, Institute for Clinical Immunology and Transfusion Medicine, Justus Liebig University Giessen, Langhansstrasse 7, 35385 Giessen, Germany; e-mail: sentot.santoso@immunologie.med.uni-giessen.de.