In this issue of Blood, Schmitt et al address the biology and safety of T cells engineered to express T-cell receptor (TCR) variants endowed with enhanced affinity for tumor-associated antigens.1
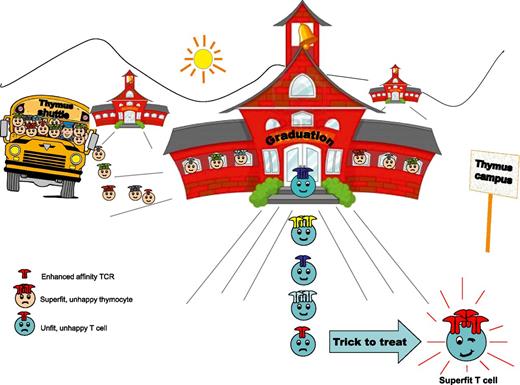
Happy thymocytes enter the thymus. Super-fit ones will be retained. Only the fit ones will graduate. Some of the unfit ones will survive too, but would not be functional in the periphery. TCR gene transfer might now be adopted to make the fit, superfit.
Happy thymocytes enter the thymus. Super-fit ones will be retained. Only the fit ones will graduate. Some of the unfit ones will survive too, but would not be functional in the periphery. TCR gene transfer might now be adopted to make the fit, superfit.
The authors hypothesize that the negative selection of high-affinity self-reactive thymocytes might overprotect against self-reactivity and result in the maturation of T cells with low-affinity TCR and suboptimal recognition of tumor-expressing, nonmutated self-antigens. This notion is supported by the finding that TCRs that bind viral nonself antigens fall within higher affinity ranges when compared with those that bind tumor-related antigens.2 As high-avidity T cells bear the potential to respond to minute antigen expression, efforts have been focused on the retrieval and/or the engineering of high-avidity tumor-specific T cells for adoptive cellular immunotherapy. Thanks to increasing knowledge of TCR engineering, it is now possible to design increased-affinity variants of any given TCR and to engineer patient T cells to recreate potent high-avidity tumor-directed T-cell responses.3 The potential to artificially increase the affinity of any given TCRs and express them on patients’ autologous lymphocytes, however, raises important questions: Could thymic mechanisms of negative selection be tricked into improving tumor recognition and, thus, the efficacy of cancer immunotherapy? And could T cells expressing an enhanced-affinity TCR bypass the affinity limits imposed by thymic selection and be safe, or are they potentially at risk to mediate autoimmune manifestations?
To begin addressing these questions, Schmitt et al engineered mature T cells with TCR variants with enhanced affinity for cognate peptides derived from WT1 and Mesothelin, two highly relevant unmutated self-antigens that are overexpressed in several tumors and are linked to oncogenesis and tumor progression.4,5 The results described show that such engineered T lymphocytes can be infused into mice without causing autoimmune tissue infiltration or damage, even after their terminal differentiation into cytolytic effectors in response to pathogenic antigen-expressing Listeria monocytogenes. Thus, limits imposed by negative selection in the thymus could be safely overcome by the genetic manipulation of mature cells (at least in the case or WT1 and Mesothelin) (see Figure). Of note, when expressed in developing precursors, using the retrogenic technique, high-affinity TCR resulted in negative selection of cells with abundant TCR levels, as well as in positive selection of low-expressing cells. In spite of surviving negative selection, however, cells expressing the enhanced-affinity TCR at low levels revealed attenuated antigen sensitivity in the periphery, supporting that the thymus imposes a given affinity threshold that cannot be overcome.6 Thus, results obtained by Phil Greenberg and collaborators indicate that although central tolerance in the thymus cannot be overruled, it can be tricked by targeting mature peripheral T cells while still avoiding autoimmunity.
This study has implications about the biology underlying negative selection in the thymus and direct consequences for adoptive T-cell immunotherapy. It elegantly demonstrates that central tolerance mechanisms can indeed be overprotective, thus limiting protective immunity (and also self-reactivity) against given nonmutated self antigens and providing an explanation for the failure to retrieve high-avidity self-reactive T cells in mice or humans. It also paves the way for more efficacious adoptive immunotherapy, providing an interesting window of opportunity for the design of better TCRs. Finally, it provocatively suggests that naive T cells with low TCR surface levels might be the ideal source for the identification of naturally occurring TCRs with an intrinsically higher affinity for tumor/self-antigens, which could then be expressed at high levels in T cells and exploited for adoptive TCR gene therapy (see Figure).
The study also leaves open questions. For instance, TCR variants tested by Schmitt et al were obtained by genetic engineering of the α chain CDR3 region. Would this be applicable to TCRs engineered in CDR1 or CDR2 loops? Would this approach be extendible to the large majority of nonmutated self-antigen? Would T cells expressing enhanced-affinity TCRs be resistant to intratumoral tolerance7,8 and result in better antitumor protection? Would a similar approach be applicable to CD4, or would this result in cells with regulatory phenotypes? Further work will certainly follow, but the results of the study indicate that it is indeed possible to trick the thymus to further the goal of treating cancer.
Conflict-of-interest disclosure: C.B. has a research contract with MolMed s.p.a. The remaining author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal