Abstract
Multiple lines of evidence suggest that invariant NKT (iNKT) cells generate an inflammatory cascade that promotes and sustains sickle cell vaso-occlusion. In prior studies of mouse models and patients with sickle cell disease (SCD), iNKT cells are increased in number and more likely to be activated compared to controls. Depleting iNKT cells in a mouse model of SCD decreases inflammation and prevents end-organ injury. NKTT120 is a humanized monoclonal antibody that specifically depletes iNKT cells. Preclinical studies show that NKTT120 has high affinity and specificity for iNKT cells. NKTT120 depletes iNKT cells in a dose-dependent manner. Return of iNKT cells to the peripheral circulation following NKTT120 administration occurs in a dose- and time-dependent manner. Our global hypothesis is that NKTT120 will deplete iNKT cells, reduce inflammation and prevent painful vaso-occlusive crises. In this phase 1 dose-escalation study, we will examine the safety of NKTT120 in steady state adults with SCD.
To determine the safety, maximum tolerated dose (MTD), pharmacokinetics, and pharmacodynamics of NKTT120 in steady state adults with SCD. The optimal dose for a phase 2 study of NKTT120 will deplete iNKT for approximately 3 months allowing for periodic dosing.
Phase 1 study utilizing a 3+3 design to evaluate single doses escalated over a range from 0.001 mg/kg to 0.1 mg/kg (0.001, 0.003, 0.01, 0.03, and 0.10 mg/kg). Primary outcome measure is safety. Secondary outcomes include pain, analgesic use, quality of life (QoL), and pulmonary function. During a screening run-in period and after dosing of NKTT120, subjects will maintain a daily smartphone eDiary (eSCaPe) to report pain, respiratory symptoms and analgesic use. ASCQ-Me and PROMIS QoL questionnaires will be administered at clinic visits. The screening run-in outcomes will be used as baseline comparison for values obtained post-dosing.
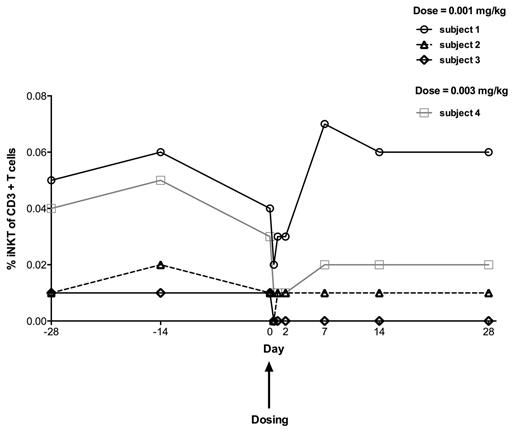
One month of follow-up data on iNKT cell numbers is available for the first four patients in the study (Figure 1). Three subjects received the lowest dose of 0.001 mg/kg and 1 subject received 0.003 mg/kg. All 4 subjects showed a reduction in the iNKT cell percent of CD3+ T cells 6 hours after NKTT120 administration. Three subjects have completed the study with iNKT cells returning to pre-dosing levels at day 7 while the remaining subject is awaiting iNKT cell recovery five weeks after dosing. The effects of NKTT120 were specific to iNKT cells as T cell, B cell and NK cell percent of lymphocytes was not affected. NKTT120 has been well tolerated with no adverse events reported.
iNKT cell percent of CD3+ T cells in 4 patients with SCD before and after NKTT120 administration
iNKT cell percent of CD3+ T cells in 4 patients with SCD before and after NKTT120 administration
In steady state adults with SCD, NKTT120 administered at the lowest dose of 0.001 mg/kg specifically reduces iNKT cells without toxicity. The dose of 0.003 mg/kg is currently being evaluated in this ongoing trial and higher doses of NKTT120 are anticipated to further deplete iNKT cells in the blood and tissue with longer times to recovery. This reduction of iNKT cells should result in a suppression of the inflammatory stimuli that promote many of the pathophysiologic sequelae seen in SCD.
Field:NKT Therapeutics: Consultancy. Eaton:NKT Therapeutics: Employment, Equity Ownership. Mashal:NKT Therapeutics: Employment, Equity Ownership. Nathan:NKT Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal