Abstract
Allogeneic stem cell transplantation (alloSCT) is the only curative treatment for most patients with CN AML. It is now well established that some relevant molecular markers can allow for the definition of risk groups after conventional chemotherapy. However, the role of these markers among other factors influencing outcome after alloSCT remains to be defined. Hence, we conducted an EBMT registry-based analysis aiming to define the exact prognostic role of molecular subgroups in conjunction with other classical risk factors after alloSCT for CN-AML.
752 adults (375 males) fulfilled the inclusion criteria, i.e. first alloSCT for CN-AML between 2000 and 2011, matched sibling (MSD) or unrelated (MUD) donor, and known mutational status of FLT3-ITD and NPM1, the two most frequently observed molecular markers in CN-AML. Median age was 51y (range, 18-71), disease status at time of alloSCT was CR1 (n=554), CR2/3 (n=90), primary induction failure (PIF, n=39), relapsed disease (n=62) and unknown (n=7). Myeloablative and reduced-intensity conditioning regimens were used in 371 and 378 patients, respectively. 597 received allogeneic PBSCs.
With a median follow-up of 27 months, The 2-year estimates of overall survival (OS) and leukemia-free survival (LFS) were respectively 68±2% and 61±6% after alloSCT in CR1, 63±2% and 53±6% in CR2/3, 36±7% and 33±8% in PIF, and 28±7% and 25±6% in relapsed disease. In 554 patients transplanted in CR1, OS, LFS, cumulative incidence of relapse (CIR) and non-relapse mortality (NRM) were calculated according to molecular subgroups. Whereas NPM1mut had no influence on outcome, FLT3-ITD was associated with increased CIR (p<0.0001), decreased LFS (p=0.0002) and OS (p=0.0006) Outcome after alloSCT of subgroups based on mutational status of NPM1 and FLT3-ITD is shown in table 1
| Table 1 . | OS (%) . | LFS (%) . | CIR (%) . | NRM (%) . |
|---|---|---|---|---|
| NPM1WT/FLT3-ITDneg (n=221) | 75±3 | 69±3 | 16±2 | 15±2 |
| NPM1WT/FLT3-ITD (n=59) | 47±7 | 44±7 | 36±7 | 19±6 |
| NPM1mut/FLT3-ITD (n=215) | 64±4 | 58±4 | 28±3 | 13±2 |
| NPM1mut/FLT3-ITDneg (n=59) | 80±5 | 77±6 | 13±4 | 11±4 |
| Global p-value | 0.0008 | 0.0006 | 0,0003 | 0,85 |
| Table 1 . | OS (%) . | LFS (%) . | CIR (%) . | NRM (%) . |
|---|---|---|---|---|
| NPM1WT/FLT3-ITDneg (n=221) | 75±3 | 69±3 | 16±2 | 15±2 |
| NPM1WT/FLT3-ITD (n=59) | 47±7 | 44±7 | 36±7 | 19±6 |
| NPM1mut/FLT3-ITD (n=215) | 64±4 | 58±4 | 28±3 | 13±2 |
| NPM1mut/FLT3-ITDneg (n=59) | 80±5 | 77±6 | 13±4 | 11±4 |
| Global p-value | 0.0008 | 0.0006 | 0,0003 | 0,85 |
.
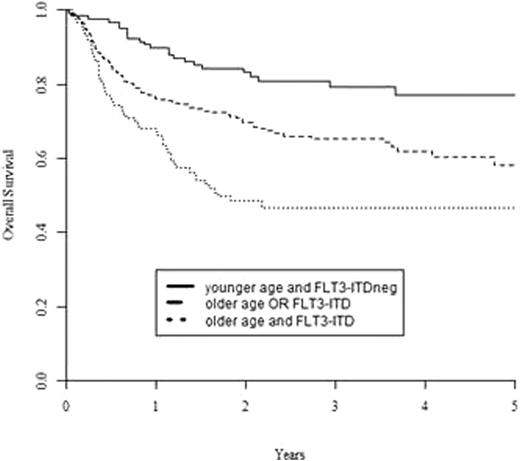
A multivariate Cox model of predefined risk factors including age, WBC at diagnosis, time to reach CR1, donor type, conditioning type (reduced vs. standard), and presence of NPM1mut and FLT3-ITD, was performed. Age >=50 years was the only risk factor for NRM (HR= 2.40, 95%CI: 1.32-4.37), while the presence of FLT3-ITD was the only risk factor for CIR (HR=2.85; 95%CI; 1.76-4.60). Both age >=50 years and FLT3-ITD were associated with inferior LFS (HR=1.65, 95%CI: 1.14-2.39, p=0.01 for age, HR=2.08, 95%CI: 1.43-3.01, p=0.001 for FLT3-ITD) and, most importantly, OS (HR=1,76; 95%CI: 1.19-2.61, p=0.005 for age, and HR=2.19; 95%CI: 1.48-3.26, p=0.0001 for FLT3-ITD). The latter data allowed to identify 3 prognostic groups with 2-years OS of 83±4% (younger age and FLT3-ITDneg), 70±4% (older age OR FLT3-ITD) and 48±5% (older age and FLT3-ITD), p<0.0001; Figure 1). On the contrary, donor type (sibling vs. MUD) or intensity of the conditioning regimen (standard vs. reduced) did not play any significant role in any molecular subgroup.
Overall survival from allo SCT for CN-AML in CR1, based on patient age and presence of an FLT3-ITD
Overall survival from allo SCT for CN-AML in CR1, based on patient age and presence of an FLT3-ITD
In the largest study on CN-AML performed thus far, we identified age >=50 years and FLT3-ITD as the major risk factors for OS after alloSCT in CR1, independently from other risk factors such as type of donor or conditioning. In patients harboring FLT3-ITD, a limited protective role for NPM1 mutation could be observed. These results underscore the importance of post-transplant strategies to prevent relapse in AML with FLT3-ITD. Encouraging results were observed after SCT in PIF, relapse or advanced CR.
Schmid:Novartis: Honoraria, Research Funding, travel grant Other; Roche: travel grant, travel grant Other; MSD: Honoraria. Rambaldi:Novartis: Honoraria; Sanofi: Honoraria; Italfarmaco: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal