Abstract
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm of hematopoietic stem cells characterized by presence of a dysregulated BCR-ABL fusion protein which leads to constitutive activation of tyrosine kinase activity. Classically, CML is treated with tyrosine kinase inhibitors (TKIs). However, TKI therapy is non-curative, and persistence of quiescent leukemia stem cells likely accounts for the inability of these agents to cure CML. β-arrestins are multifunctional adapter proteins which regulate G protein-coupled receptor (GPCR) signaling and trafficking and have recently been identified as mediators of distinct cellular signaling cascades independent of G proteins. β-arrestins also play a role in the Smoothened/Hedgehog pathway, as well as in the Wingless/Frizzled (Wnt/Fz) signaling axis, both of which have been associated with the development of CML. We, therefore, hypothesize that β-arrestin2 (βarr2) is necessary for the development and propagation of CML and may function as a therapeutic target.
Demonstrate that loss of βarr2, using an inducible conditional knockout mouse model, slows progression of CML.
We used a standard murine retroviral transduction system to model chronic phase BCR-ABL positive CML. KLS cells (Lin-, Sca-1+, c-kit+) were harvested from bone marrow of donor C57BL6/J βarr2F/F-CreERT2+/- (Cre positive, CD45.2) and age matched C57BL6/J βarr2F/F-CreERT2-/- (Cre negative, CD54.2) male mice. KLS cells were retrovirally transduced with MSCV-BCR-ABL-IRES-GFP and were subsequently injected retro-orbitally into sublethally irradiated congenic wild type recipient male mice (CD45.1). Donor mice were engineered using global Cre-ER/loxP technology in order to induce site-specific recombination and loss of βarr2 when treated with tamoxifen. Mice who received Cre positive cells lost βarr2 only in hematopoietic cells. Recipient mice were treated with tamoxifen 75mg/kg daily via intraperitoneal injection for 5 days starting day 3 after transplant and were monitored for signs of leukemia development. Weekly blood analysis included WBC count, number of donor cells by flow cytometry, blood film, and qPCR for BCR-ABL expression. Survival was compared between animals receiving Cre positive (βarr2F/F-CreERT2+/-) and Cre negative (βarr2F/F-CreERT2-/-) donor cells as well as both tamoxifen treated and untreated conditions.
Treatment of donor C57BL6/J βarr2F/F-CreERT2+/- mice with tamoxifen resulted in decreased βarr2 expression within 10 days in multiple tissues including spleen and bone marrow. By day 10, βarr2 expression was 9.5 ± 3.0% in Cre positive mice relative to pretreatment expression levels (Figure 1).
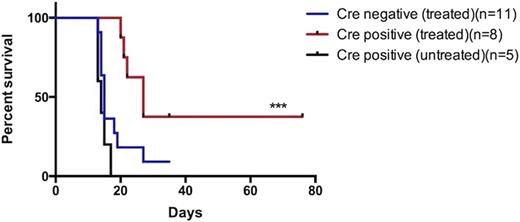
In total, 8 mice received Cre positive cells and 11 mice received Cre negative cells. Ten of 11 (90.9%) Cre negative mice developed CML as evidenced by splenomegaly, leukocytosis, increased BCR-ABL expression measured by qPCR and increased number of donor cells detectable by flow cytometry. Median survival was 15 days. Six of 8 (75%) Cre positive mice developed disease with median survival of 27 days (HR 3.2, 95% CI; 1.525-12.2, p=0.013)(Figure 2). At day 11, flow cytometry for donor CD45.2 cells present in peripheral blood of recipient mice was 38 ± 2.05% in Cre negative versus 16.5± 3.9% in Cre positive mice (p<0.0001). Spleen size at death and WBC count at day 11 were not significantly different between groups.
These data demonstrate that targeting βarr2 prolongs the course of disease in chronic phase CML and raise the possibility that loss of βarr2 after disease onset may lead to disease regression. βarr2 may therefore represent an alternative therapeutic target for CML independent of tyrosine kinase inhibition.
(A) Percent β arr2 expression over time in spleen is significantly lower in Cre positive versus Cre negative mice (*** p <0 .0001 by two-way ANOVA)(n=3 mice at each time point on days 8, 10 and n=2 at each time point day 12). (B) Percent βarr2 expression at day 12 in spleen relative to pretreatment expression levels (*** p=0.0002 by t-test)(n=2 at each time point).
(A) Percent β arr2 expression over time in spleen is significantly lower in Cre positive versus Cre negative mice (*** p <0 .0001 by two-way ANOVA)(n=3 mice at each time point on days 8, 10 and n=2 at each time point day 12). (B) Percent βarr2 expression at day 12 in spleen relative to pretreatment expression levels (*** p=0.0002 by t-test)(n=2 at each time point).
Kaplan-Meier Curve. Survival of Cre positive mice treated with tamoxifen was statistically significantly higher compared to Cre negative treated mice by log-rank (Mantel-Cox) test (HR 3.2, 95% CI; 1.525-12.2, ***p=0.013).
Kaplan-Meier Curve. Survival of Cre positive mice treated with tamoxifen was statistically significantly higher compared to Cre negative treated mice by log-rank (Mantel-Cox) test (HR 3.2, 95% CI; 1.525-12.2, ***p=0.013).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal