Abstract
Ponatinib targets the inactive conformation of the ABL1 kinase and avoids interacting with the side chain of the mutated 315 residue. In vitro, ponatinib inhibits all single-point BCR-ABL1 mutations. Yet, a significant proportion of patients with chronic myeloid leukemia in chronic phase (CML–CP) do not respond to ponatinib and a subset loses their response during the course of treatment. The mechanisms of resistance to ponatinib are currently not well characterized.
To determine the impact of compound BCR-ABL1 mutations (polymutants) on the activity of ponatinib.
BCR-ABL1 mutational status was determined in 70 pts with CML-CP post imatinib failure and during dasatinib therapy by DNA expansion of specific clones followed by DNA sequencing of ≥10 clones. Free energy of binding (DGbind) for the unmutated and all mutant BCR-ABL1 kinase/inhibitor complexes were obtained using Molecular Mechanics/Poisson-Boltzmann Surface Area (MM-PBSA) methodology. Single and polymutant BCR-ABL1 alleles obtained by direct mutagenesis and their expression was forced into Ba/F3 cells by electroporation by the pMX/eGFP-BCR-ABL1 expression vector using the Amaxa System.
After imatinib failure, 125 ABL1 kinase domain mutations at 113 amino acid positions were detected in 61/70 (87%) pts, including 38 (54%) with mutations in ≥20% of sequenced clones. Mutations conferring resistance to >1µM imatinib were detected in 30 (43%) pts. Polymutant BCR-ABL1 alleles were detected in 29/70 (41%) pts. These patients received dasatinib for a median of 19 mos (range, 2-52), during which dasatinib-resistant mutations were detected in 10/32 (31%) assessable cases (5 with T315I). Polymutants were present in 16/32 (50%) pts (all of them dead in blast phase). The proportion of clones carrying unmutated BCR-ABL1 was markedly lower in patients who only achieved a minor or no cytogenetic response compared to those achieving a major cytogenetic response (p=0.0001), suggesting exhaustion of unmutated clones and expansion of mutant (and polymutant) clones linked to clinical dasatinib resistance.
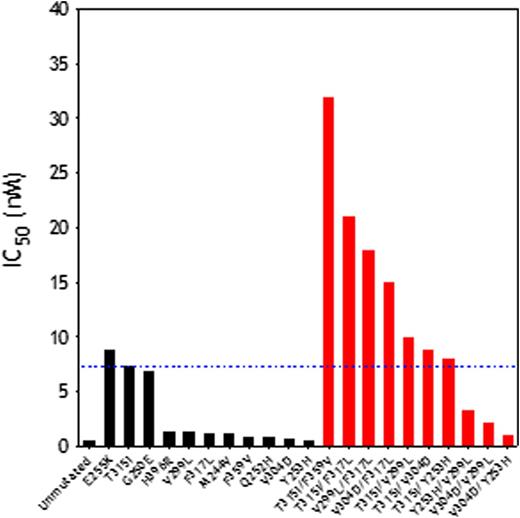
Then, we performed 3D structural analyses to determine the thermodynamic impact of 21 BCR-ABL1 mutants (11 single and 10 double mutants) in the ability of ponatinib to bind the kinase domain (Table). Most single mutants did not result in high ponatinib resistance (except for E255K, IC50=8.8nM; DGbind=-10.99±0.01). However, the association of any 2 of 3 point mutants (T315I, F317L, V299L) in a dual polymutant produced highly resistant BCR-ABL1 proteins that exhibited fold change values from 19 to 40, compared to the unmutated protein, with T315I/F359V displaying the highest resistance (IC50=61nM; DGbind=-10.23±0.03 kcal/mol), unveiling a mechanism of escape to ponatinib.
In Ba/F3-based assays, ponatinib (but not imatinib or dasatinib) was active against Ba/F3-BCR-ABL1T315I cells. Polymutants exhibited very high ponatinib resistance (10-fold higher than that of cells carrying BCR-ABL1T315I). As predicted in silico, BCR-ABL1T315I/F359V was the most resistant polymutant tested. Cell growth inhibition was coupled with CrkL and STAT5 phosphorylation inhibition. Ponatinib, while suppressing STAT5 phosphorylation, could not suppress CrkL phosphorylation in cells expressing the BCR-ABL1T315I/F359V polymutant kinase, even at 100 nM (50-fold the IC50 required to inhibit BCR-ABL1T315I).
Polymutants are very frequent in pt samples after TKI failure (particularly after sequential TKI therapy) and tend to induce high ponatinib resistance. Our in silico platform predicted very accurately TKI sensitivity in cells carrying different BCR-ABL1 mutations, which makes it clinically applicable for matching specific mutations to the most effective TKI. Some polymutants require ponatinib concentrations not clinically reachable, thus representing a mechanism of escape to ponatinib therapy through selection and expansion of refractory clones.
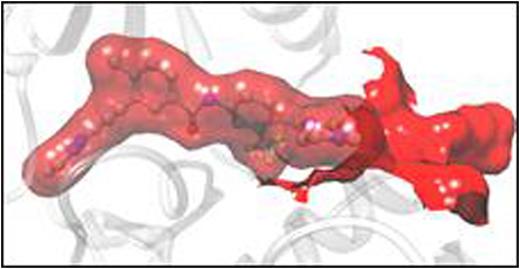
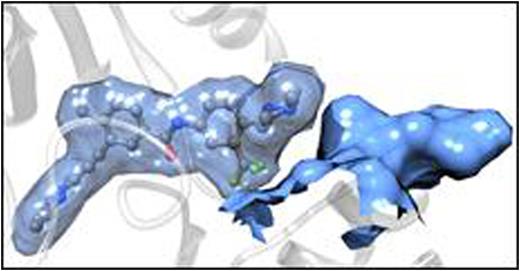
(A) Activity of ponatinib against single (black columns) and polymutant (red columns) BCR-ABL1 proteins. (B) The dual mutant T315I/F359V induces a rearrangement of the ABL1 binding pocket that results in weaker ponatinib binding with respect to the unmutated protein.
(A) Activity of ponatinib against single (black columns) and polymutant (red columns) BCR-ABL1 proteins. (B) The dual mutant T315I/F359V induces a rearrangement of the ABL1 binding pocket that results in weaker ponatinib binding with respect to the unmutated protein.
Talpaz:ariad: Research Funding. Cortes:Ariad: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Teva: Consultancy, Honoraria, Research Funding. Quintas-Cardama:ariad: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal