Abstract
Rationale. Improved outcome of female patients treated with rituximab (R) has been reported for first-line diffuse large B-cell lymphoma (DLBCL) (Müller C, et al. Blood 2012; 119: 3276-84), maintenance treatment of relapsed DLBCL (Gisselbrecht C, et al. J Clin Oncol 2012; 30: 4462-9), and induction as well as maintenance treatment of follicular lymphoma (Jäger U, et al. Haematologica 2012; 97: 1431-8; Salles G, et al. Lancet 2011; 377: 42-51.). This sex-specific response has been attributed to lower body weight or a smaller volume of distribution of the drug in women. Rituximab maintenance in DLBCL and FL grade 3 in complete remission (CR) or CR unconfirmed (CRu) after R-CHOP-like therapy did not significantly prolong EFS or PFS in the NHL13 trial conducted by the Austrian Study Group (AGMT) (http://www.clinicaltrials.gov/ct2/show/NCT00400478?term=ML+18223&rank=1). However, a trend for improved EFS (p value = 0.067), a 44% reduction of relapses, as well as a particularly good outcome in patients with an International Prognostic Index (IPI) of 0 or 1 was noted (Jaeger U, et al. Hematol Oncol 2013; 31 (Suppl. 1): 96-150 (abstract 119)). In the second interim analysis imbalances in toxicity between male and female patients treated with R were observed. This prompted us to analyse sex specific outcome in the final analysis.
Patients and Methods. In the NHL13 multicenter, prospective trial 683 previously untreated adult patients with DLBCL (N=662) or FL G3B (N=21) were randomized for R maintenance (375 mg/m2 every 2 months for 2 years) (N=338) or observation only (N=345) between June 2004 and September 2008. Patients at all clinical stages in CR or CRu after treatment with 4 to 8 cycles of R-CHOP like therapy were included. Median age was 58 years (range 19-88) with 48.2% or 52.8% male patients in the R or observation arms, respectively. Median observation time from inclusion was 45 months. The primary endpoint of this study was EFS. Secondary endpoints included PFS, OS and safety. Data were analysed using a Cox regression model.
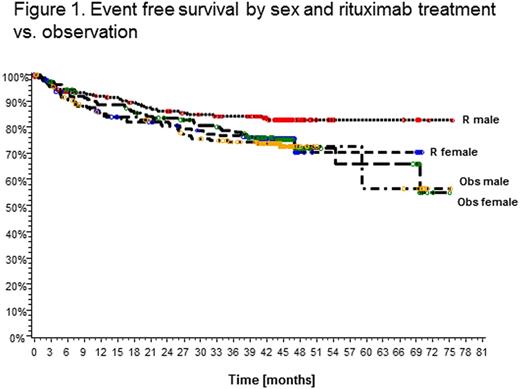
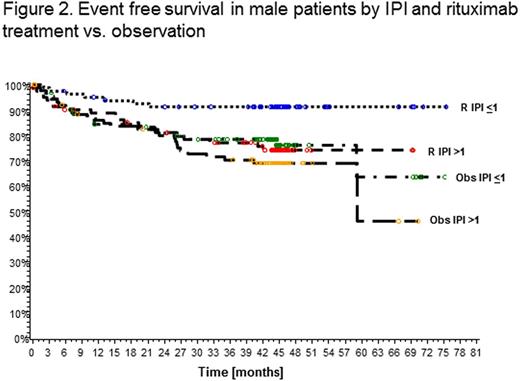
Results. Event free survival in male patients was superior in the rituximab maintenance treatment arm compared to observation (EFS at 3 years 84.1% vs. 74.4%; HR 0.58, 95% CI 0.36-0.94; p=0.0267) while no difference was observed in female patients (EFS at 3 years 76.8% vs. 78.7%; HR 1.5, 95% CI 0.67-1.66; p=0.8246) (Fig.1).The effect was particularly pronounced in male patients with low IPI (<1) (EFS at 3 years 91.2% vs. 78.2%), while this benefit was not as strong in male patients with IPI>1 (77.1% vs. 70.2%) or women with IPI<1 (79.5% vs. 82.5%) or >1 (74.7% vs. 75.7%) (Fig.2). In a multivariate analysis of factors associated with EFS in male patients R treatment together with age (<60 vs. >60 years), continent (Africa, America, Asia, Australia, Europe) and clinical stage (1/2 vs. 3/4) remained independent, while this was not observed in female patients. When IPI was included as a separate variable in the model, R treatment was the only independent factor in men. Similar analysis conducted for PFS showed a significant effect of R in men (PFS at 3 years 89.0% vs. 77.6% for observation; HR 0.45, 95% CI 0.25-0.79; p=0.0058) but not in women (83.7% vs. 80.5%; HR 0.79, 95% CI 0.47-1.33; p=0.3693). Again, R treatment and age were independent factors in multivariate analysis in men. PFS in patients with IPI<1 treated with R at 3 years was 96.1% in men vs. 89.2% in women. No significant differences in OS were observed at this time. Of note, more female patients receiving R maintenance had at least one adverse event CTC grade 3/4 (21.7% vs.12.3% in males, p=0.0297). Infections and infestations (all grades) occurred most frequently in female patients in the R arm (40.6% vs. 29.4% (male R) vs. 33.7% (male obs.) vs. 26.4% (female obs.); p=0.0341).
Conclusion. This unforeseen subgroup analysis by sex from a large randomized prospective trial provides surprising but clear evidence that 2-monthly R maintenance for 2 years significantly improves EFS and PFS of male patients with DLBCL (and FL G3B). Maintenance therapy may be compensating for underdosing in men with current R containing induction regimens and indicates the necessity to prolong or increase rituximab dosing for men with aggressive B-cell lymphoma, particularly in the low IPI subgroup.
Jaeger:Roche: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Off Label Use: Rituximab as maintenance treatment for DLBCL. Trneny:Roche: Honoraria, Research Funding. Fridrik:Roche: Honoraria. Hedenus:Vifor Pharma: Consultancy, Honoraria. Thaler:Roche: Honoraria, Research Funding. Keil:Roche: Honoraria. Dittrich:Roche: Honoraria, Research Funding. Greil:Roche: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Goldstein:Roche: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal