Abstract
Hairy cell leukemia (HCL) is a chronic lymphoproliferative disorder recently found to be characterized by somatic BRAFV600E mutations. The malignant cell in HCL exhibits features consistent with a mature B-lymphocyte, including cell-surface expression of the pan-B-cell marker CD19 and monotypic surface immunoglobulins with clonal rearrangements of immunoglobulin heavy and light chains. Despite possessing these stereotypic features, the cell of origin of HCL has been long debated, and no cell type along the continuum of developing B-lymphocytes has been definitively identified as the normal counterpart of HCL cells.
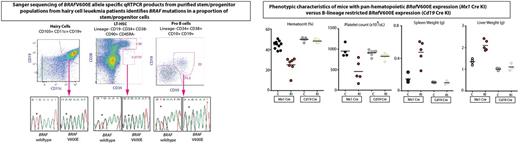
Given the human HSC genetic and functional cell data, we conditionally expressed BRafV600E from its endogenous locus at different stages of hematopoiesis, including in HSPCs and committed B cells. Mice with conditional expression of BRafV600E in Mx1Cre+ BRafV600E knock-in mice died of a lethal hematopoietic malignancy characterized by features of human HCL including splenomegaly, anemia, thrombocytopenia, increased circulating sCD25, and increased clonogenic capacity of B-lineage cells (evidenced by infinite serial replating in the presence of IL-7) (Figure ). This disorder was transplantable into lethally-irradiated recipient mice. In contrast, mice with expression of BRafV600E restricted to the B-cell lineage with Cd19 Cre manifested no overt malignant phenotype up to one year of age. Stimulation of these mice with alloantigen through injections of sheep red blood cells resulted in germinal center B-cell hyperplasia, but still did not result in development of a clonal B-cell proliferation.
Recent case reports have noted that refractory HCL patients respond to mutant BRAF inhibition with vemurafenib. We investigated the effect of vemurafenib on HSPCs and hematopoiesis in patients treated on a phase II study of the mutant BRAF inhibitor vemurafenib for relapsed/refractory HCL as well as in our in vivo murine models. Flow cytometric analysis of bone marrow cells from vemurafenib treated HCL patients revealed normalization of HSPC frequencies within three months of starting therapy, concomitant with an improvement in peripheral blood counts. Consistent with this, evaluation of the in vitro clonogenic capacity of sorted LT-HSC's from the bone marrow of HCL patients revealed a significant increase in myeloid/erythroid colony formation in HCL patients treated for 3 months with vemurafenib compared to their pretreatment marrows. Likewise, treatment of wildtype mice transplanted with Mx1Cre+ BRafV600E mutant bone marrow cells revealed improvement in anemia and hepatosplenomegaly with in vivo therapy.
Overall, these findings link the pathogenesis of HCL to a specific somatic genetic abnormality present in HSCs and provide evidence that mature B-cell malignancies can initiate in the HSC compartment. Moreover, these data suggest that the use of therapies targeting MAP kinase signaling in HCL may lead to durable remissions not only by eliminating the mature leukemic cells but also through targeted inhibition of signaling and survival in HCL initiating cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal