Abstract
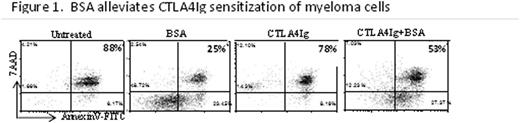
Despite novel chemotherapeutic regimens, myeloma patients invariably develop drug resistance and eventually die due to disease relapse, highlighting the necessity for newer therapies to extend survival. Interactions with bone marrow stroma are essential for drug resistance and survival in myeloma (MM). Not much is known about the specific cell types or molecular interactions that mediate this. Clinical studies have shown that expression of CD28 (the prototypic T cell costimulatory molecule) on MM correlates with disease progression. Our recent publication and abstracts (ASH2012 #722, ASH2011 #147, ASH2010 #132) show that interaction with dendritic cells (DC) in the bone marrow provide pro-survival signals to MM through CD28 via interactions with its ligands CD80/CD86 on DC or CD86 on other MM. Clinical studies have separately shown that CD28 or CD86 expression on MM portends poor survival in myeloma patients. In our previous ASH abstracts, we have shown that blocking MM CD28 interactions with CD80/CD86 on DC or other MM using novel reagents such as CTLA4Ig (Abatacept®, a recombinant fusion protein between CTLA4 and human IgFc) or blocking αCD28(Fab) fragments can reduce CD28 mediated survival in MM. In contrast, activating CD28 on MM with agonistic antibodies improves survival against different therapeutics or serum starvation. Another mechanism by which cancer cells survive exogenous stress is via redox regulation and our data that show that BSA (a known scavenger of reactive oxygen species (ROS)) can alleviate CTLA4Ig mediated sensitization of MM cells (Fig 1) suggest involvement of redox regulation in CD28 mediated MM survival. Intrinsic oxidative stress is a hallmark of cancer and is associated with abnormal cancer growth and progression. Others have reported that cancer cells adapt to intrinsic oxidative stress by developing enhanced anti-oxidant capacity, and are more resistant to exogenous stress. Literature also suggests that cancer cells that have higher intrinsic oxidative stress are also more likely to be sensitive to any disruption of redox regulation than normal cells. In MM, flow cytometric analysis using ROS dye CM-DCFDA show significantly higher (8-9 fold) basal ROS levels in the drug resistant U266 as opposed to the drug sensitive cell line MM.1S (Fig 2). Thioredoxin (TRX1) is a key ROS induced anti-oxidant protein essential for redox regulation and survival in many types of cancers. But not much is known about its role in myeloma survival. Interestingly, gene expression analysis of public datasets of plasma cells from normal, MGUS and myeloma patients show significant increases in the levels of TRX1 with disease progression. Further, within the myeloma patient group, TRX1 levels were significantly higher in the “relapsed” group relative to new or smoldering MM groups (Fig 3). In contrast, the levels of thioredoxin-interacting protein (TXNIP), a negative regulator of thioredoxin was significantly lower in myeloma patients and in the relapsed group, compared to normal or new diagnosed patients respectively (Fig 3). Expression trends for TRX1 and TXNIP were inversely correlated across patient groups. While CD28 activation in MM can alleviate drug induced cell death in myeloma, it could not overcome cell death induced by the specific TRX1 inhibitor PX-12 (Biomira Inc, currently under Phase 2 clinical trials for pancreatic cancer). Moreover, viability assays show that high-ROS cell line U266 was 4 fold more sensitive to PX-12 than the low-ROS MM.1S cells which is interesting since U266 is drug resistant and is thus representative of relapsed myeloma. ROS assays with PX-12 on MM show rapid dose dependent increases in ROS levels in U266 cells, while it was much lower in MM.1S suggesting a rationale for the higher sensitivity of U266. This was reversed when a ROS scavenger N-acetyl cysteine (NAC) was added. NAC also completely abrogated PX-12 mediated apoptosis in both myeloma cell lines U266 and MM.1S suggesting that PX-12's activity was solely via disruption of redox regulation. Our data reveal an important redox regulatory mechanism mediated by thioredoxin which play a supportive role for CD28 mediated survival in myeloma and disease relapse, disruption of which could selectively target relapsed drug resistant myeloma.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal