Abstract
The presence of circulating plasma cells (PCs) in multiple myeloma (MM) is a known poor prognostic marker. Prior studies have utilized a technically challenging slide-based immunofluorescence technique to detect and quantify the presence of circulating PCs which was not widely adapted in clinical practice. More recently, the routine use of flow cytometry has provided the opportunity to quantitatively assess circulating PCs in MM patients with relative ease. We report the prognostic value of quantifying circulating clonal PCs using multiparametric flow cytometry in MM patients with relapsed disease.
We evaluated all MM patients seen at the Mayo Clinic, Rochester from 2009 to 2011 with previous or ongoing relapsed disease and who had their peripheral blood samples evaluated by flow cytometry. Each blood sample had its peripheral blood mononuclear cells isolated by ficoll gradient and stained with antibodies to CD45, CD19, CD38, CD138 and cytoplasmic Kappa and Lambda Ig light chains. A six-color multi-parameter flow cytometer (Becton Dickinson FacsCantos II) was used to examine each sample with a target of detecting 150,000 events (cells) that was then analyzed using the Facs Diva Software. Plasma cells were selectively analyzed through combinatorial gating using light scatter properties and CD38, CD138, CD19, and CD45. Normal PC's were then separated from clonal plasma cells based on the differential expression of CD45, CD19 and polytypic Ig light chains. The clonal plasma cells detected were reported out as the number of clonal events/150,000 collected total events. For those samples where less than 150,000 events were gated or examined, the number of final clonal events was adjusted to 150,000 events. Survival analysis was performed by the Kaplan-Meier method and differences in survival assessed using the log rank test.
There were 647 consecutive patients with a history of treated MM who had a peripheral blood flow cytometry as part of their routine clinical evaluation. The median patient age was 62 years (29-88) and 55% were male. The median time since diagnosis was 12 months (range: 1-363) and the median number of lines of treatment received was 2 (1-11). There were 81 (13%) patients with clonal circulating PCs with a median of 368 cells (4 – 133,464). The 2-year overall survival (OS) for the 81 (13%) patients with any circulating PCs was 17% compared with 65% for those with none (P<0.001). The presence of circulating clonal PCs was associated with high-risk disease by FISH (P <0.001) as well as higher PCLI (P <0.001). We then correlated the presence of circulating clonal PCs with the disease status at flow cytometry assessment. Among the study patients, only 145 (22%) had actively relapsing disease with the remaining 502 (78%) in a plateau including patients in CR. Circulating clonal PCs were more likely to be detected in patients with actively relapsing disease compared with plateau phase (43% vs. 4%, P < 0.001); none of the CR patients and <5% of the remaining plateau phase patients had any detectable circulating clonal PCs.
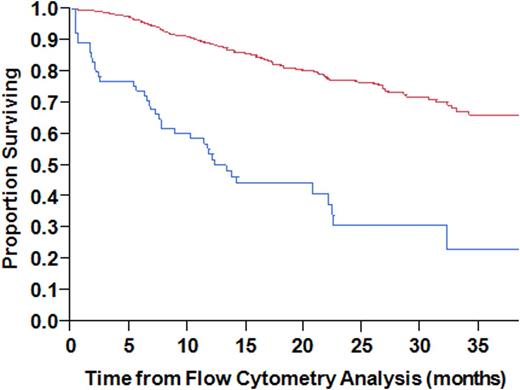
We then restricted the analysis to the actively relapsing patients; the best cutoff predicting 1-year mortality by ROC analysis was 100 events. Based on this, we defined >=100 events as a cutoff for defining the prognostic role of circulating clonal PCs in actively relapsing patients. The median OS for those with >=100 clonal PC events was 12 months compared with not reached for those with <100 events (p<0.001; Figure 1
). Among patients with actively relapsing disease, >= 100 circulating PCs was associated with a higher ISS stage, plasma cell labeling index and bone marrow PC% compared with those with <100 circulating PCs. In a multivariable model, only LDH > 222 (HR: 2.08, P = 0.045) and >= 100 circulating PCs (HR: 2.48, P = 0.048) were found to adversely affect OS in actively relapsing patients.
The utilization of flow cytometry to quantify circulating clonal PCs in patients with relapsed MM appears to have significant prognostic relevance. In patients with actively relapsing disease, >= 100 circulating PCs predicted for worse OS with a median of 12 months. Future studies are needed to determine if this would allow an opportunity to develop a more risk adapted approach for patients with relapsed disease.
Kumar:Celgene: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Onyx: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

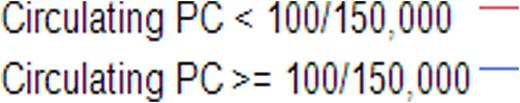
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal