Abstract
Durable responses to lenalidomide (LEN, an immunomodulatory drug, IMiDTM) occur in both non-del5q and del5q Myelodysplastic Syndrome (MDS). In non-del5q MDS and multiple myeloma, modulation of the bone marrow microenvironment by an unknown mechanism has been theorized. IMiDs initiate numerous effects on the immune system, some of which may induce an anti-leukemic and anti-inflammatory response. The connection between the drug's immune modulating activity and its hematopoietic stimulatory activity in MDS is currently untested. We and others have shown that LEN expands T-cells and increases IL-2 (p<0.001) by substituting for CD28 co-stimulation. In support of this, we found that LEN activates a CD28-specfic transcription factor pCREB in the absence of extracellular ligation of the CD28 receptor suggesting that it is acting to suppress a pharmacologic target that is involved specifically in costimulatory signaling. Normally, several negative regulators provide immunotolerance to T-cells to limit self-reactivity and protect against autoimmune disease. Anti-cancer immunosurveillance is often circumvented through acquired resistance to costimulation.
The critical role of immune modulation in LEN-induced hematologic response was examined by testing the performance of a predictive biomarker in patients stratified by del5q status in a training cohort and in a blinded validation cohort. Molecular analyses were performed on sorted populations of cells and knock-down of genes in normal human T-cells. Finally, we demonstrate the relevance of cereblon, a molecular target of LEN, in the negative regulation of costimulatory CD28 signaling using a homozygous germline knockout mouse.
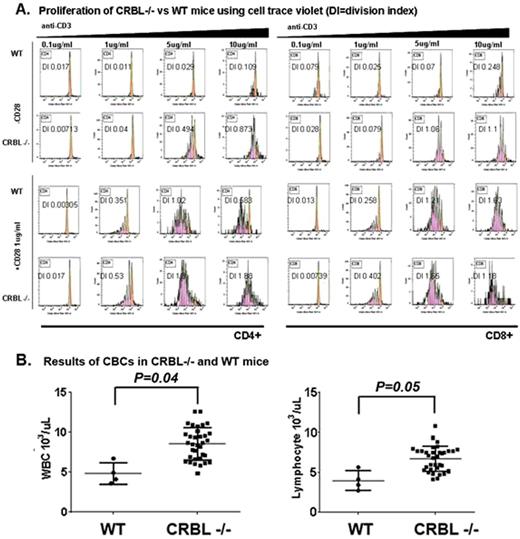
Comparison of sorted CD28+CD8+ and CD28-CD8+ human T-cells illustrates the importance of the expression of this receptor in LEN-induced T-cell activation. CD28- T-cells were found to be resistant to LEN. Accumulation of CD28- cells is a form of accelerated immunosenescence that is often associated with cancer and has been reported in MDS. To determine if CD28- T-cell accumulation is associated with LEN hematopoietic outcome in patients, we performed T-cell profiling before treatment in a training cohort (n=21) and a blinded validation cohort (n=35). Of the T-cell markers studied, a higher percentage of CD28- T-cells was associated with LEN hematopoietic failure in the training cohort (p=0.001). Using a R-Part-defined cutoff of CD28+ vs CD28- T-cells, the biomarker sensitivity was found to be 86%, specificity 82%, positive predictive value (PPV) 67%, and negative PV (NPV) 93% in non-del5q MDS patients, but did not improve response discrimination in del5q MDS. The association between CD28 and LEN response led us to hypothesize that the molecular target of LEN is involved in signaling by the CD28 receptor. A landmark study identified cereblon (CRBL), a RING-domain E3-Ubiquitin Ligase (UbL) to be the primary target of thalidomide and LEN. To examine the effect of CRBL on CD28 signaling, germ-line crbl knock-out mice (crbl-/-) were examined compared to wild-type (WT) C57BL6 mice. We found that crbl deficiency augments T-cell proliferation (Fig. 1A) and contextual activation of IL2 in the absence of CD28 co-stimulation similar to LEN. Examination of the bone marrow, thymus, and peripheral blood shows that crbl is a negative regulator of lymphopoiesis in the spleen and thymocyte with increased peripheral blood lymphocyte counts (Fig. 1B) and increased double negative (DN)-1 (P=0.01), DN3 (p=0.03), and reduced DN4 (p=0.005) thymocytes in crbl-/- compared to WT mice.
Our results indicate that immune reconstitution mediated by T-cell CD28 signaling is important for hematologic response in non-del5q MDS, but not del5q MDS, and is consistent with different molecular mechanisms of action in these two groups of patients. LEN may improve T-cell function by directly suppressing CRBL a novel E3-UbL regulator involved in lymphopoiesis, thymocyte development and CD28 co-stimulation. Our data suggests that CRBL acts similarly to the E3 UbL proteins Cbl-b, GRAIL, and ITCH that mechanistically induce immunotolerance. In response to LEN, T-cell functional activation may sustain anti-leukemic immunosurveillance or act to remove suppressive immature cell populations from the bone marrow microenvironment allowing hematologic improvement in non-del5q MDS.
Epling-Burnette:Celgene: Research Funding. McDaniel:Celgene: Employment. List:Celgene: Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal