Abstract
Polymorphonuclear cells (PMNs) roll along vessel walls by binding to E- and P-selectins on the endothelial surface. These vascular adhesion molecules are involved in the initial tethering of PMNs on endothelium rather than their firm adhesion. Endothelial cell–expressed P- and E-selectins interact with leukocyte counterreceptors P-selectin glycoprotein ligand-1 (PSGL-1) and E-selectin ligand-1 (ESL-1). These glycoprotein counterreceptors are active only when modified posttranslationally by fucosylated oligosaccharides represented by the sialyl Lewis x tetrasaccharide (sLex) or its structural variants. Because of the important role that fucose plays in protein glycosylation and cellular functions, specific inhibitors of its incorporation could have significant applications in basic research and therapy. One such inhibitor, 2-fluorofucose peracetate, was recently reported to block fucosylation of cellular proteins and to inhibit binding of PMNs to P- and E-selectin. One potential application is the inhibition of leukocyte recruitment in pro-inflammatory disease states such as sickle cell disease (SCD). P-selectin expression on endothelium has been demonstrated to play an important role in vaso-occlusion, a hallmark of SCD. Inhibition of P-selectin binding inhibits vaso-occlusion in transgenic sickle mice. Therefore, we hypothesized that 2-fluorofucose would inhibit tissue inflammation and vaso-occlusion in SCD mice.
NY1DD sickle mice were given plain water or 20 or 100 mM 2-fluorofucose in their drinking water ad libitum. The first cohort of mice (n=8/group) was treated for 7 days. On day 7 the mice were sacrificed with CO2, an EDTA blood sample was collected from the heart and the liver was removed and frozen in liquid nitrogen. Total white blood cell counts and differentials were performed by manual counting using a hemocytometer and Wright-stained blood smears, respectively. Nuclear extracts were prepared from nuclei isolated from liver homogenates. Western blots of the nuclear extracts were immunostained with antibodies to NF-ĸB phospho-p65 a marker of NF-ĸB activation. The second cohort of mice (n=4/group) was also treated for 7 days. On day 4, dorsal skin fold chambers were implanted onto the mice. On day 7, flowing venules in the dorsal skin-fold chamber window were selected and mapped using intravital microscopy. Thereafter, the mice were infused via the tail vein with human stroma-free hemoglobin (0.32 µmols heme/kg), a known inducer of vascular stasis in SCD mice. At 1 and 4 hours after infusion the same venules were re-examined and the percentage of vessels that had become static (no flow) was recorded. A positive control group of NY1DD mice (n=3) with implanted dorsal skin-fold chambers was given water to drink and pretreated with hemin (40 µmols/kg X 3 days, i.p.), a known inhibitor of vascular stasis.
White blood cells counts were 16.3±3.2 (K/µL, mean±SD) in SCD mice treated with water. The white counts increased to 22.1±5.2 in SCD mice treated with 20 mM 2-fluorofucose and 34.2±7.2 in SCD mice treated with 100 mM 2-fluorofucose, respectively (p<0.05 for all pairwise comparisons). NF-ĸB in liver and other organs is activated in SCD mice compared to normal C57BL/6 mice. Nuclear NF-ĸB phospho-p65 was partially diminished in mice treated with 20 mM 2-fluorofucose and markedly decreased in mice treated with 100 mM 2-fluorofucose or pretreated with heme (Fig. 1).
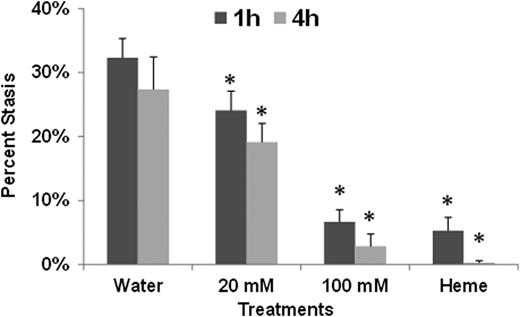
Infusion of hemoglobin induced ∼30% microvascular stasis at 1 and 4 hours in SCD mice treated with water (Fig. 2). Treatment with 20 mM 2-fluorofucose partially inhibited stasis at 1 and 4 hours (p<0.05 compared to water). Treatment with 100 mM 2-fluorofucose or heme inhibited stasis to 6.7% or less at 1 and 4 hours (p<0.025 compared to water).
Oral administration of 2-fluorofucose has significant anti-inflammatory and anti-vaso-occlusive properties in SCD mice.
Belcher:Seattle Genetics, Inc.: Research Funding. Chen:Seattle Genetics, Inc.: Research Funding. Nguyen:Seattle Genetics, Inc.: Research Funding. Okeley:Seattle Genetics, Inc.: Employment. Senter:Seattle Genetics, Inc.: Employment. Benjamin:Seattle Genetics, Inc.: Employment. Vercellotti:Seattle Genetics, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal