Abstract
Allogeneic stem cell transplant (SCT) remains the only curative option for patients with β thalassemia major (TM). Graft rejection has been a major problem in this group of patients. The increased risk of graft rejection has often been attributed to the number of blood transfusions that these patients are exposed to. However, the published data is confusing with one large series suggesting that patients who had >100 transfusion pre-SCT had a significantly lower risk of graft rejection (Blood 1996; 87: 2082). It is also generally accepted that in patients with thalassemia intermedia the risk of alloimmunisation (red cell) is reduced if transfusion is initiated <12 months of age (Vox Sang 1990; 58:50). We undertook a retrospective analysis to study the impact of age at first transfusion on graft rejection among patients with TM who received an allogeneic SCT at our center.
From October, 1991 to April, 2013, 400 HLA matched related transplants for TM was done at our center. The median age was 8 years (range: 1-24) and there were 250 (62.5%) males. 154 (38.5%) were Lucarelli Class II and 229 (57.2%) were in the Class III risk group. Majority (72%) received a busulfan based conditioning regimen while 22% received a treosulfan based regimen. Bone marrow was the source of stem cells in 81% and PBSC in the rest. Majority of the patients received a CSA plus short course methotrexate GVHD prophylaxis regimen. There were 11 (2.8%) early regimen related toxicity (RRT) deaths prior to day 12 and these were excluded for analysis of graft rejection. Of the remaining 389 cases there were 48 (12.3%) graft rejections. Among these 26 (54%) were primary graft failures while 22 (46%) were secondary graft failures. The median time to a secondary graft failure was 122 days (range: 40 - 2210). The median age at first transfusion in this cohort was 6 months (range: 1-66; data not available in 10). The median number of blood transfusions prior to SCT was 85 (range: 4 – 450).
Baseline characteristics and comparison of patients that had a graft rejection versus those that did not after excluding early regimen related toxicity deaths (< day 12).
| . | Had graft rejection N (%) / Mean±SD/ Median(Range) . | Did not have graft rejection N (%) / Mean±SD/ Median(Range) . | Cox regressionUnivariate analysis . | Cox regressionMultivariate analysis . |
|---|---|---|---|---|
| N | 48 | 341 | P-value | P-value |
| Age (years) | 8 (2-19) | 7 (1-24) | NS | |
| Sex: M | 28 (58.3) | 217 (63.6) | NS | |
| Class III | 32 (66.7) | 187 (54.8) | NS | |
| Live size (cm) | 4 (1-14) | 3 (0-12) | 0.006 | 0.019 |
| Female donor to male recipient | 15 (31.2) | 126 (37) | NS | |
| Splenectomy | 8 (16.7) | 35 (10.3) | 0.083 | |
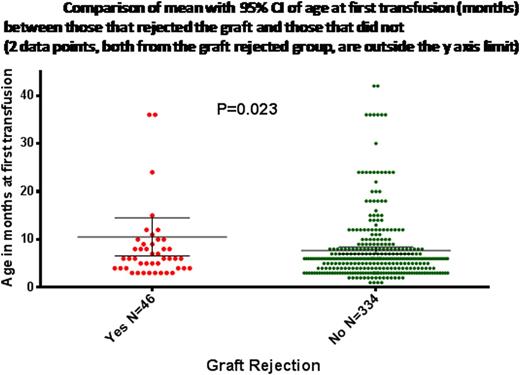
| Age at first transfusion (months) | 10.5±13.4 | 7.7±6.7 | 0.036 | 0.022 |
| Total number of transfusions prior to SCT | 100 (6-373) | 85 (4-450) | NS | |
| Conditioning regimen | - | - | NS | |
| Busulfan based | 32 (80) | 246 (76.2) | ||
| Treosulfan based | 8 (20) | 77 (23.8) | ||
| Stem cell source | - | - | NS | |
| BM | 44 (91.7) | 272 (79.8) | ||
| PBSC | 4 (8.3) | 69 (20.2) | ||
| CD34 cell dose | 9.6 (2.35-15) | 9 (2 – 30) | NS |
| . | Had graft rejection N (%) / Mean±SD/ Median(Range) . | Did not have graft rejection N (%) / Mean±SD/ Median(Range) . | Cox regressionUnivariate analysis . | Cox regressionMultivariate analysis . |
|---|---|---|---|---|
| N | 48 | 341 | P-value | P-value |
| Age (years) | 8 (2-19) | 7 (1-24) | NS | |
| Sex: M | 28 (58.3) | 217 (63.6) | NS | |
| Class III | 32 (66.7) | 187 (54.8) | NS | |
| Live size (cm) | 4 (1-14) | 3 (0-12) | 0.006 | 0.019 |
| Female donor to male recipient | 15 (31.2) | 126 (37) | NS | |
| Splenectomy | 8 (16.7) | 35 (10.3) | 0.083 | |
| Age at first transfusion (months) | 10.5±13.4 | 7.7±6.7 | 0.036 | 0.022 |
| Total number of transfusions prior to SCT | 100 (6-373) | 85 (4-450) | NS | |
| Conditioning regimen | - | - | NS | |
| Busulfan based | 32 (80) | 246 (76.2) | ||
| Treosulfan based | 8 (20) | 77 (23.8) | ||
| Stem cell source | - | - | NS | |
| BM | 44 (91.7) | 272 (79.8) | ||
| PBSC | 4 (8.3) | 69 (20.2) | ||
| CD34 cell dose | 9.6 (2.35-15) | 9 (2 – 30) | NS |
In conclusion, delay in the onset of transfusion in patients with β thalassemia major undergoing a HLA matched related allogeneic SCT probably has a greater adverse effect on engraftment than the total number of transfusions prior to SCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal