Abstract

Hematopoietic progenitor cells (HPCs) collected by apheresis of G-CSF-stimulated donors have surpassed bone marrow as the predominant graft source for hematopoietic stem cell transplantation in adults. Among healthy allogeneic donors, demographic characteristics (age, sex, and BMI) and baseline hematologic parameters have been shown to affect HPC mobilization, leading to significant variability in peak levels of CD34 cell egress into the blood and in quantity of CD34 cells harvested by apheresis. Racial differences in G-CSF-mediated HPC mobilization are less well characterized. Benign physiologic neutropenia is common among healthy African Americans (AAs), and may be due to decreased stem cell reserve, fewer G-CSF receptors, or Duffy (null) blood group antigen-mediated decrease in leukocyte trafficking into the circulation. However, preliminary studies have shown relatively robust CD34+ cell mobilization among non-Caucasians given G-CSF (Vasu et al., Blood 2006).
We retrospectively analyzed 1,096 consecutive healthy allogeneic related and unrelated first-time donors who self-characterized their race as AA or Caucasian. They underwent G-CSF (filgrastim, Neupogen, Amgen) stimulated HPC collection by leukapheresis from April 1999 to May 2013. G-CSF dose ranged from 10-16 mcg/kg, given daily for 5 days. An unstimulated leukapheresis procedure for lymphocyte collection was performed in the 7 days preceding G-CSF in 336 subjects. Apheresis procedures were performed on the CS-3000 Plus or COBE Spectra device. Baseline lab data included CBC, serologic blood group antigen typing, and Hb electrophoresis in AA donors. CD34+ cell counts were performed on peripheral blood immediately pre-apheresis (2 hours after the 5th dose of G-CSF) and on the apheresis product. Values are given as mean ± SD.
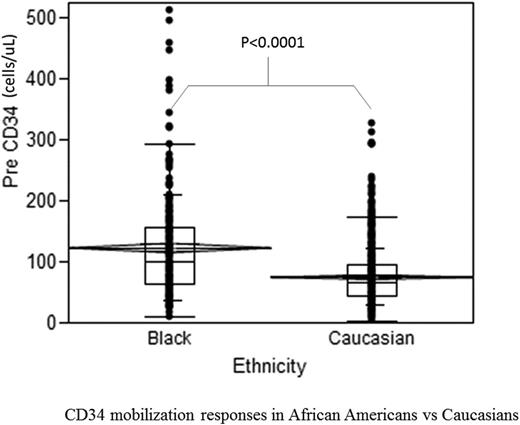
All AA (n=215) and Caucasian donors (n=881) with complete data sets were included. Sex ratio was similar among the groups (45 vs 52% male; p=0.09). AAs were younger (39 vs 43 yrs, p=0.001) and had greater weight (86 vs 81 kg, p=0.001) and BMI (30 vs 27; p<0.0001) than Caucasians. G-CSF dose/kg was similar in the 2 groups, but total daily dose of G-CSF was greater in AAs than Caucasians (920 vs 850 mcg, p<0.0001). After adjusting for age, sex, height, weight, and total daily G-CSF dose, peak CD34+ cell mobilization immediately pre-apheresis was higher among AAs than Caucasians (123 ± 87 vs 75 ± 47 cells/uL; p<0.0001) (Figure). When laboratory parameters such as baseline WBC, MNC, and platelet counts were included in the stepwise regression model, AA race remained a significant predictor of higher peak CD34 cell counts. At higher G-CSF doses (16 mcg/kg/d), the difference in mobilization responses between the 2 groups was less apparent (peak CD34 counts 123 vs 93 cells/uL, AA (n=33) vs Caucasian (n=73), p=0.07) than at lower doses (10 mcg/kg/d), where peak CD34 counts were 123 vs 74 cells/uL, AA (n=182) vs Caucasian (n=808), p<0.0001. AAs had lower baseline ANC (3.4 vs 4.0 x 103 cells/uL, p<0.001) than Caucasians, but demonstrated significantly higher peak WBC and MNC counts after G-CSF. In AA donors with known HbS status, presence of sickle cell trait had no effect on CD34 mobilization (peak CD34 counts 123 ± 91 vs 107 ± 72 cells/uL, HbAS (n=41) vs HbAA (n=84), p=0.34). Similarly, in AA donors with known Duffy phenotype, Duffy expression did not affect CD34 mobilization (peak CD34 counts 114 ± 81 vs 134 ± 85 cells/uL, Fya-b- (n=49) vs Fya+ &/Fyb+ (n=20), p=0.4). Lymph-apheresis prior to starting G-CSF was associated with significantly improved CD34+ cell mobilization; however the effect did not differ by race. CD34 apheresis yield was also greater in AAs than Caucasians (51 ± 35 vs 32 ± 21 x 106 cells per liter processed, p <0.0001), consistent with higher pre-apheresis counts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal