Abstract
BEACOPPescalated is the standard treatment for advanced Hodgkin Lymphoma (HL) within the German Hodgkin Study Group (GHSG) and other cooperative groups. Bleomycin and vincristine cause significant acute and long-term toxicity and are frequently discontinued during the course of therapy. However, the impact of dose reduction of these drugs on outcome and tolerability of BEACOPP has not been systematically assessed. Thus, we performed a retrospective analysis of patients treated within the GHSG trials HD12 (8xBEACOPPescalated versus 4xBEACOPPescalated plus 4xBEACOPPbaseline) and HD15 (8xBEACOPPescalated versus 6xBEACOPPescalated versus 8xBEACOPP14) trials for advanced stages.
Based on the intention-to-treat analysis, characteristics and outcome of patients receiving the full number of cycles of HD12 and HD15 were analyzed with respect to bleomycin and/or vincristine discontinuation. Progression-free survival (PFS) and overall survival (OS) were estimated from end of chemotherapy according to the Kaplan-Meier method and compared between groups using the log-rank test. To compare differences in patient characteristics, Fisher's exact test was used for rates and Wilcoxon's rank-sum test was used for continuous parameters. Receiver operating characteristic (ROC) analyses were performed in patients who had an event or were followed for at least 2 years after therapy.
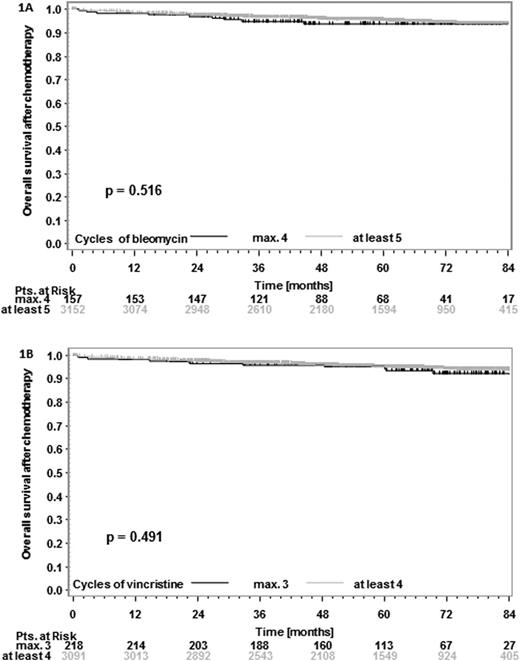
3309 (89.4%) of patients received the full number of planned cycles and had complete chemotherapy documentation available. Bleomycin was discontinued in 10.5% and vincristine in 21.7% of cases. All other drugs had discontinuation rates less than 1.5%. 157 (4.7%) of patients received ≤4 cycles of bleomycin and 218 (6.6%) of patients received ≤3 cycles of vincristine; these were compared to patients with >4 cycles of bleomycin (3152 patients [95.3%]) and >3 cycles of vincristine (3091 patients [93.4%]). Discontinuation of bleomycin or vincristine was more frequent in patients aged 50 or older (4.4% and 7.4% with ≤4 cycles of bleomycin in patients <50 and ≥ 50 years, respectively, p=0.001; 6.2% and 9.5% with ≤3 cycles of vincristine in patients <50 and ≥ 50 years, respectively, p=0.015). More female patients had vincristine discontinued (8.4% and 5.5% female and male patients, respectively, with ≤3 cycles, p=0.001) whereas there was no significant difference between female and male patients regarding bleomycin discontinuation (p=0.6). After a median follow-up of 59 and 67 months for PFS and OS, respectively, there was no significant PFS or OS difference in patients with ≤4 or >4 cycles of bleomycin (6-year PFS-difference 0.3% [95%CI -5.7 to 6.2%]; 6-year OS-difference 0.4% [95%CI -3.7 to 4.5%]; Figure 1A). Similarly, there was no significant PFS or OS difference in patients with ≤3 or >3 cycles of vincristine (6-year PFS-difference -1.6% [95%CI -6.4 to 3.2%]; 6-year OS-difference 1.9% [95%CI -2.5% to 6.3%]; Figure 1B). To assess if the number of bleomycin and vincristine cycles received had an impact on 2-year PFS and OS, ROC analyses were performed. However, failure could not be predicted irrespective of the number of cycles chosen as cutpoint. Detailed analyses and comparisons of patient characteristics, dose delivery of chemotherapy and toxicity will be presented.
Bleomycin and vincristine discontinuation due to drug-specific side effects seemed to be safe in this setting. Given the limitations of this retrospective analysis, our data suggest that bleomycin and vincristine may have a limited role in the BEACOPP regimen.
von Tresckow:Novartis: honoraria for acting as a consultant: Consultancy; Takeda Pharma GmbH: reimbursement of congress, travel, and accommodation costs and honoraria for preparation of scientific educational events: Honoraria. Böll:Celgene: Travel Grant Other. Borchmann:Millenium The Takeda Oncology Company: Research Funding; Takeda Pharma GmbH: Travel Grants, Travel Grants Other. Engert:Seattle Genetics, Inc.: Honoraria, Research Funding; Millennium: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal