Abstract
Childhood acute myeloid leukemia (AML) is characterized by chromosomal instability and requires intensive therapy for cure. Here we present a large-scale study from 3 Children's Oncology Group (COG) trials to define the genomic architectural profiles of pediatric AML and to describe whole-arm gains and losses and focal rearrangements using SNP microarrays, accounting for mutation status, non-neoplastic cell infiltration and aneuploidy. We also investigate the association between somatic copy number aberrations (CNAs) and event free survival (EFS).
A total of 505 matched tumor-remission samples from 459 children with de novo AML were obtained from the COG studies AAML-0531, AAML-03P1, and CCG-2961. 254 paired tumor-remission samples were genotyped on the Affymetrix SNP 6.0 chip at the University of Washington, Seattle, WA, and 251 on the Illumina 2.5M OmniQuad at the Children's Hospital of Philadelphia, PA. All genotyping output was converted to log R ratio and B-allele frequency values and annotations updated to hg19, GRCh37. Illumina intensities were additionally corrected for patterns of genomic wave.
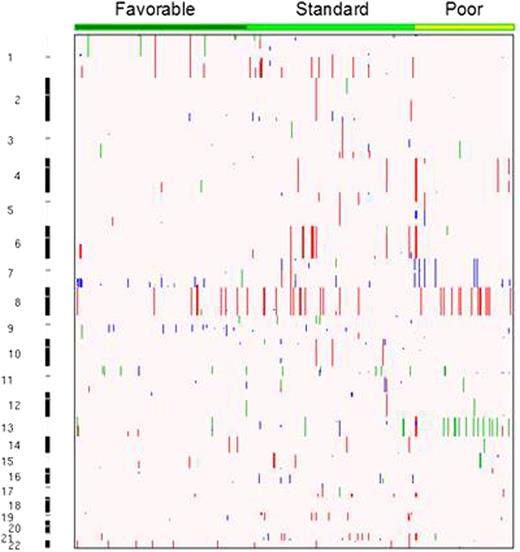
A matched allele-specific copy number analysis of tumors (ASCAT) was performed using ASCAT 2.2. A total of 246 (98%) Illumina and 247 (97%) Affymetrix samples passed quality control criteria. ASCAT profiles of patients genotyped on both platforms were manually reviewed and those with highest false CNA calls excluded. Samples with a low signal-to-noise ratio were either eliminated or visually annotated. All CNA segments were manually inspected and false positives removed. The resulting 452 ASCAT profiles were stratified into the risk categories 1) favorable, e.g. inv(16), t(16;16), t(8;21), NPM1, and CEBPα, 2) standard, e.g. normal karyotype, +8, +21, +22, del(7q), del(9q), abnormal 11q23, or other structural changes, and 3) poor, e.g. -5, -7, del(5q), or FLT3/ITD+. GISTIC 2.0 was used to identify genomic areas with significant recurrent aberrations, e.g. amplifications, deletions, and copy-neutral loss-of-heterozygosity (CN-LOH) events. Finally, we investigated whether profiles of genomic instability in the AML genome are predictive of 3 year EFS by using the total number of CNAs as a measure for allelic imbalance.
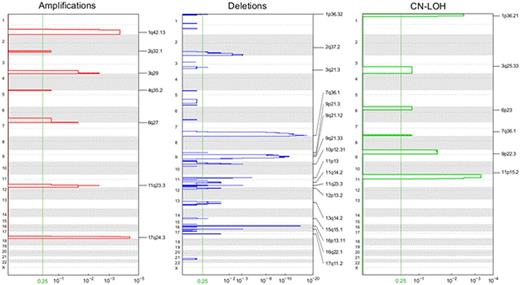
The inter-platform concordance for samples genotyped on the two platforms was very high. On average leukemic samples acquire 1.14 somatic CNAs, with a mean of 1.1, 1.3, and 0.8 in the favorable, standard, and poor risk groups respectively (Fig 1). CN-LOH events are observed in 14% (n = 64) of the patients, with 28% involving chromosome 13, and others involving the arms of 11p (23%), 1p (11%), 9p (8%), 7q (6%), 19q (6%), and 3q (5%). Known mutations in AML were enriched in recurrent focal CNA regions, as shown by amplifications on 17q24 (*TK1), 1q32, 3q28, 11q23 (*MLL), 6q27 (*DLL1), 2q32.1, and 4q35.2 (Fig 2). Focal CN-LOH regions were confined to 11p15.5 (*NUP98, *PICALM, *WT1), 1p36.3 (*RUNX3, *NRAS), 9p24.3 (*MLLT3), 3q25.3, 6p23 and 7q35 (*MLL3). Deletions include 7q36.1 (*MLL3, *EZH2), 16p13.11 (*MYH11), 9q21.32, 11p13 (*WT1), 2q37.1 (*IDH1, *DNMT3A), 10p12.31 (*MLLT10), 11q23.3 (*MLL), 16q22.1 (*CBFB), and 1p36.3 (*RUNX3). The association between CNA status and 3 year EFS approached statistical significance for all patients (Table 1), and EFS was significantly lower in standard risk patients with CNAs (51% no CNA vs 34% with CNAs, P 0.03). CNA status did not alter the event risk in the other risk groups.
Prognostic Properties of CNAs
| Risk category . | CNA . | Total . | 3yr EFS . | Logrank P . |
|---|---|---|---|---|
| Overall | 0 | 215 | 54% ± 7% | 0.087 |
| 1+ | 235 | 47% ± 7% | ||
| Favorable | 0 | 80 | 66% ± 11% | 0.809 |
| 1+ | 87 | 65% ± 10% | ||
| Standard | 0 | 80 | 51% ± 11% | 0.030 |
| 1+ | 100 | 34% ± 10% | ||
| Poor | 0 | 48 | 42% ± 15% | 0.376 |
| 1+ | 41 | 37% ± 15% |
| Risk category . | CNA . | Total . | 3yr EFS . | Logrank P . |
|---|---|---|---|---|
| Overall | 0 | 215 | 54% ± 7% | 0.087 |
| 1+ | 235 | 47% ± 7% | ||
| Favorable | 0 | 80 | 66% ± 11% | 0.809 |
| 1+ | 87 | 65% ± 10% | ||
| Standard | 0 | 80 | 51% ± 11% | 0.030 |
| 1+ | 100 | 34% ± 10% | ||
| Poor | 0 | 48 | 42% ± 15% | 0.376 |
| 1+ | 41 | 37% ± 15% |
The number of CNAs occurring in this cohort is lower than previously reported. However, the presence of somatic CNAs in standard risk patients is significantly associated with a worse treatment outcome that is similar to the poor risk group. This study confirms the established regions of CNA enrichment in pediatric AML and identifies novel regions that may involve driving mediators of tumor fitness and/or acquired resistance to targeted therapies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal