Abstract
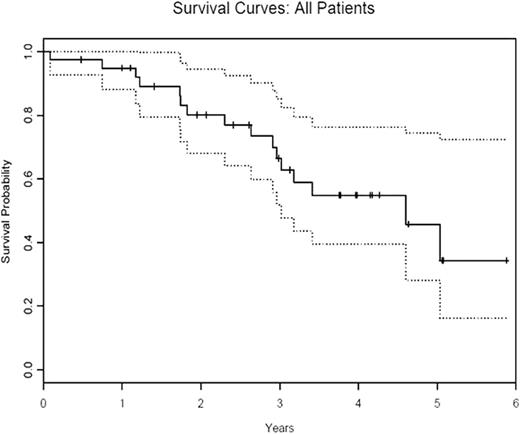
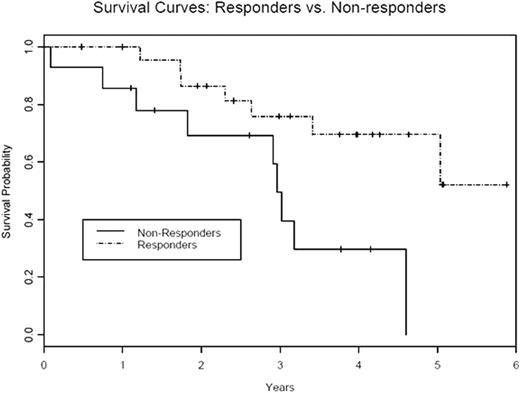
In a subgroup of patients with myelodysplastic syndrome (MDS) an immune mechanism is believed to cause bone marrow failure. In these individuals immunosuppressive treatment (IST) can restore marrow function. We previously reported a 68% response rate in a phase I/II study of alemtuzumab in 31 patients with low to int-2 risk MDS (Sloand etal JCO Dec 10, 2010:5166-5173). Patients were enrolled according to their predicted probability of response based on an algorithm that incorporated HLA-DR15 positivity, age and transfusion burden (Blood 2003 Oct 15; 102(8):3025-7). We now report updated efficacy data on our cohort of 40 patients and long term follow up data on responders. The first 37 patients received 10mg of alemtuzumab for 10 days intravenously. Alemtuzumab was administered subcutaneously in the subsequent 3 patients. Primary endpoints were changes in peripheral blood counts. Secondary endpoints included improvement in the transfusion requirements (in transfusion- dependent patients), duration of response, and late effects of treatment, relapse and survival. Thirty nine patients were evaluable. One patient discontinued treatment after one dose because of severe hypotension and was excluded from analysis. Median follow-up time was 25 months (3-64 months). Median age was 56 years (23-71 years). The overall response rate (ORR) was 64% (25/39) with 21% of patients having a complete response (8/39). Twenty of 29 (69%) int-1 patients; 4 of 7 (57 %) int-2 patients and 1 of 3 (33%) low risk patients responded following alemtuzumab treatment. Median time to response was 3 months with median duration of response 9 months (range 1-69 months). Eight of 11 patients who relapsed regained their response with addition of cyclosporine and one patient was lost to follow up. The median duration of response in patients rescued with cyclosporine is 37 months (1-42 months). The median duration of response to immunosuppressive treatment (alemtuzumab +/- cyclosporine) is 22.2 months (range 3-69 months). All responding patients who were previously transfusion dependent became transfusion independent after alemtuzumab treatment. One of three patients who received subcutaneous alemtuzumab administration responded. The median survival for all patients was 34 months (range 1-72 months) (figure 1a) with median survival of 36 months (6-72 months) in responding group compared to 16 months (1-56 months) in non-responding group (p<0.001) (figure 1b). Five of twelve patients with abnormal cytogenetics at the start of treatment had a complete cytogenetic remission at one year. One patient with monosomy 7 and another with del 13 were in cytogenetic remission for 4.5 years and 4 years respectively. Decline in their blood counts correlated with re-emergence of initial clone. Three patients with del 13, 5q- and del 5 continue to remain in cytogenetic remission at 4, 2 and 1 years respectively. The absolute neutrophil count at baseline was significantly associated with overall survival on univariate analysis. On multivariate analysis, factors significantly associated with overall survival were male sex, bone marrow hypocellularity/normocellularity, prior immunosuppressive treatment, baseline absolute neutrophil count and platelet count. All patients became lymphopenic after alemtuzumab administration and most patients continued to be lymphopenic at 3 years. Baseline absolute lymphocyte count, ratio of CD4/CD8 cell count, absolute CD4 and NK cell counts and kinetics of lymphocyte recovery were not predictive for response. Alemtuzumab was generally well tolerated with most patients having manageable infusion reactions with the first dose. Sixteen of the 39 patients became transiently positive for EBV DNA and 6 of 29 CMV seropositive patients had reactivation but no patient developed clinically significant viral disease. Alemtuzumab is well tolerated and is effective in a subset of patients with MDS. Long term responses with cytogenetic remissions are possible. Patients who relapse can be rescued with cyclosporine. Immunosuppresion should be considered in patients likely to respond based on a simple algorithm and alemtuzumab is an alternative to ATG based regimens.
Off Label Use: Alemtuzumab use as immunosuppresive agent in myelodysplastic syndrome.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal