Abstract
Current guidelines for treating patients with thromboembolism and concomitant thrombocytopenia are based on anecdotal data and experts’ opinion. We previously described an in vitro assay using thromboelastography (TEG) to evaluate the effects of anticoagulants on plasma clot formation in the presence of autologous platelets. We showed that specific TEG clotting profiles for plasmas with predefined platelet counts in the presence of fondaparinux, rivaroxaban and dabigatran were less compromised compared with clots containing unfractionated heparin (UFH) and dalteparin. In this study, we further characterize the combined effects of anticoagulants and low platelet counts on the architecture of fibrin clots in plasma.
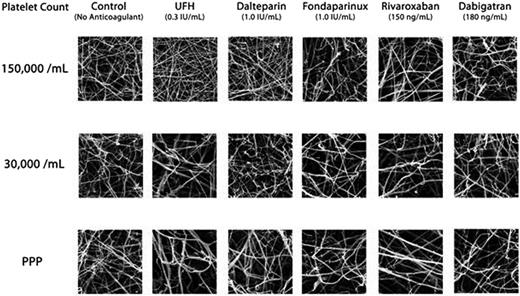
To determine the effect of various anticoagulants on the surface structure of fibrin clots in plasma when generated with autologous platelets at varying levels.
Scanning electron microscopy (SEM) was used to assess the surface structure of clots. Fresh human platelet-rich plasma and platelet-poor plasma (PPP) were obtained from the same donor and then the platelets were subsequently added back to PPP so that the final plasma samples contained predefined levels of platelets (PPP, 30,000, and 150,000 /mL). Each reaction mixture contained 30 µg/mL corn trypsin inhibitor, and one of the following anticoagulants: unfractionated heparin (UFH 0.3 IU/mL), dalteparin (1.0 IU/mL), fondaparinux (1.0 IU/mL), rivaroxaban (150 ng/mL) or dabigatran (180 ng/mL). For each condition, clotting was initiated with 10 mM CaCl2 and 1.2 pM tissue factor on a 0.02 µm Millipore membrane. After a 3-hr incubation, the clots were washed with phosphate buffered saline (pH 7.4), fixed with 2% v/v glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), and then stained with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer. The samples were dehydrated with gradient ethanol series, critical point dried, mounted onto stubs, and then gold sputter-coated for SEM examination at 20,000×. The porosity, fibril width and the number of fibrils were quantified for each clot generated.
In the absence of anticoagulants, reducing the amount of platelets from 150,000 /mL to <10,000/mL in PPP had minimal effect on the porosity of clots, although there was a reduction in the number of fibrils by approximately 30% in the PPP sample (p < 0.05) and the fibrils were generally thicker (Fig. 1). Addition of anticoagulants to plasma containing 150,000/mL of platelets minimally changed the clot structure, except for UFH and dalteparin, which significantly increased the number of fibrils. Anticoagulants profoundly changed the clot structure as platelet levels fell below 150,000/ml. The greatest effects were observed for UFH, followed by dalteparin and then fondaparinux. The changes included an increase in porosity of clots (p < 0.05), a reduction in the number of fibrils (p < 0.05), and an increase in the width of fibril strands (p < 0.05). For the newer factor-specific anticoagulants, rivaroxaban changed the clot structure similar to fondaparinux in low platelets conditions, but dabigatran changed the clot structure less than rivaroxaban.
SEM images of plasma clot formed with varying levels of platelets in the absence or presence of anticoagulants.
SEM images of plasma clot formed with varying levels of platelets in the absence or presence of anticoagulants.
The present study supports our previous TEG observations that anticoagulants intensified the negative effects of low platelets during the formation of fibrin clots. This SEM study also demonstrates that anticoagulant therapy, in particular UFH or dalteparin, substantially changes the fibrin clot structure. This information provides evidence supporting the current clinical practice of withholding anticoagulants for patients with platelet count <30,000/mL. In addition, these in vitro data suggest that fondaparinux and the new factor-specific oral anticoagulants may be safer because clot structure is better preserved when compared to the traditional heparinoids. Yet, the absence of an antidote for these anticoagulants is a major hurdle in testing this hypothesis clinically because patients with severe thrombocytopenia inherit a high risk of bleeding.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal