Abstract
Patients (pts) with thalassemia major (TM) require regular red blood cell transfusions. Adequate iron chelation prevents morbidity and mortality due to transfusional iron overload, and must be guided by accurate assessment of tissue iron levels. Magnetic resonance imaging (MRI) can non-invasively measure liver iron content (LIC) and cardiac iron, and has almost entirely supplanted liver biopsy for LIC at our center. The therapeutic goal is to either (a) maintain iron status within a consensus target range, or (b) decrease the iron burden in pts above the target. Three chelators are FDA approved in the US: deferoxamine (DFO), deferasirox (DFX), and deferiprone (DFP), (approval years 1968, 2005 and 2011 respectively). The aim of this study was to evaluate our ability to improve iron status over time in the MRI and oral chelator era.
This IRB-approved, single-center, retrospective observational study covered the period from Jan 2005, when MRI iron assessments became standard at our center, to Dec 2012. The study population included all TM pts followed for chelation at our center who had >2 MRI studies during the study period. LIC was measured by calculating T2* and, starting April 2006, also by measuring T2 using the commercial Ferriscan® technique. Liver T2* was converted to LIC using a regression equation (Wood et al. Blood, 2005; 106:1460). Cardiac iron concentration was measured by calculating cT2*; in this abstract both T2* in msec and its reciprocal R2* (1000/cT2* in Hz, which varies proportionally to iron) are reported. The target for LIC was <7 mg/g dry wt (dw), (mean of T2* and Ferriscan LIC) and for cardiac iron, cR2*<50 Hz (i.e. cT2* >20 msec). Statistical analyses were performed in SAS.
42 pts (55% male) met the inclusion criteria and had a median age at first MRI of 17.5y (range 1.9-43). Over a mean follow-up period of 5.2±1.9 y, 190 MRIs were performed with median of 4.5 MRIs per pt, interquartile range 3-6. In 2005, DFO was the predominant chelator (70% vs 26% on research use of chelators, DFX; n=27); DFX predominated after its commercial launch. 29/40 (73%) were on DFX by 2009, but this proportion dropped to 23/36 (64%) by 2012. 13/42 pts (31%) remained within the target ranges for cardiac T2* and LIC throughout the study period. 29/42 pts (69%) had at least one cardiac T2* or LIC out of the target range in a total of 97 MRIs. 38/97 (40%) of these out-of-range MRIs prompted a change in chelation strategy: 61% dose change only, 34% change of monotherapy agent, and 5% change from monotherapy to combination. Two pts died of heart failure due to iron overload during the study period; both had taken DFP before their deaths, but for divergent duration (3 days vs 5 y).
The median number of chelation changes was 1.4 per pt/y (IQR 0.9-1.9). 175/229 (76%) dosing changes were for iron status as assessed by MRI or ferritin; 7/229 (3%) were dose decreases for side effects, and 2% were due to weight change only.
Change in chelators occurred 82 times during the study. 34% of chelator changes were due to low or high iron status by MRI or Ferritin. 11% of changes were for side effects to a prior chelator and 54% were for other reasons (commercial launch of DFX or clinical trials).
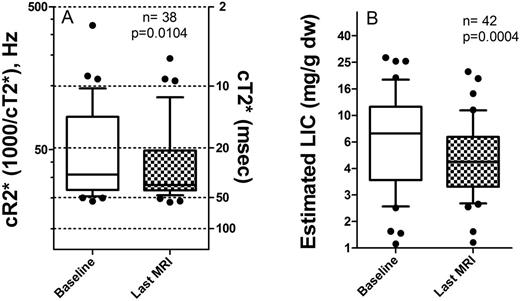
From initial to final MRI, both LIC and T2* status of our pts improved significantly (figure). At the initial MRI, 16/41 (40%) of pts were in target range for both LIC and cR2*, 4/41 (10%) were in the highest (undesirable) range of LIC>15 mg/g dw, and/or cardiac T2* <10 msec.
From first to last cardiac T2* assessment (n=38), 63% of pts started and ended within the target range, 13% improved from abnormal to target range, 24% remained out of the target range. The two pts who died were among the persistent abnormal cardiac T2* group. For LIC (n=42), 45% remained in the target range throughout, 33% started out of target range and ended within, 12% improved but not to the target, 7% worsened, and one outlier remained severe.
The introduction of routine MRI assessments of LIC and cardiac R2* (T2*), together with the introduction of oral chelators, has improved the fraction of TM pts with liver and cardiac iron within the target range at our center. Annual MRIs facilitate chelation changes when necessary.
Legend: A: Cardiac iron status from first to last MRI for each subject. Reciprocal cR2* and cT2* are on left and right Y-axes. B: Liver iron status. P-values are by Wilcoxon signed-rank test.
Neufeld:Shire: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Apopharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal