Abstract
Bortezomib and high-dose melphalan was associated with a higher complete remission (CR) rate in a phase II trial by the IFM group. Four doses of bortezomib 1 mg/m2 IV were given on days -6, -3, +1 and +4. (Roussel M et al. Blood 2010. 115:32-37).In a randomized phase II trial we reported that the addition of bortezomib to a preparative regimen of arsenic trioxide (ATO), ascorbic acid (AA), and melphalan followed by autologous hematopoietic stem cell transplantation (auto-HSCT) failed to demonstrate a statistically significant improvement in CR rate or survival in patients (pts) with newly diagnosed or relapsed multiple myeloma (Sharma M et al. Cancer. 118:2507-15).This study was limited by a short follow up (median 36 months) and inclusion of pts with relapsed and refractory disease. In this retrospective analysis, we evaluated the long-term (5-years) impact of the addition of bortezomib to this preparative regimen in pts who underwent auto-HCT in first remission or with primary refractory disease.
Forty-three pts underwent auto-HCT in first remission or with primary refractory disease. All patients received ATO 0.25 mg/kg intravenously on days -9 to -3, AA 1000 mg intravenously on days -9 to -3, and melphalan 100 mg/m2 intravenously on days -4 and -3. Pts received either no bortezomib (n=13), or bortezomib at a dose of either 1 mg/m2 of 1.5 mg/m2 on days -9, -6, and -3. The primary endpoint was CR rate and secondary endpoints were progression free survival (PFS) and overall survival (OS).
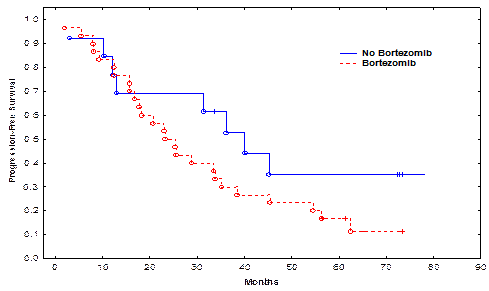
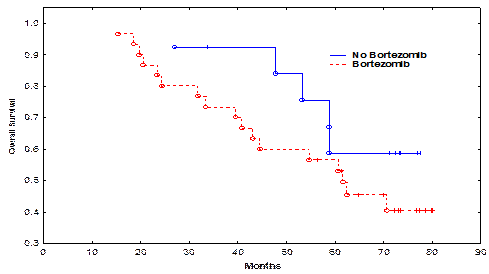
Pt characteristics are shown in the attached Table. The median follow-up for all surviving pts was 61.4 months (range, 15-80 months). Pts in the bortezomib and non-bortezomib arms were matched for age, gender, high-risk cytogenetic abnormalities, time to auto-HSCT, use of bortezomib in induction, and disease burden at auto-HSCT (Table). Only 5 (17%) pts in the bortezomib and 4 (30%) in the non-bortezomib group received maintenance therapy. There was no significant difference in the time to neutrophil engraftment or treatment-related mortality TRM between the bortezomib and non-bortezomib groups. The CR rate in the bortezomib group and the non-bortezomib group was 20% and 53%, respectively (p=0.03). There was no significant difference in rates of CR + very good partial remission (VGPR), or CR + VGPR + partial remission (PR) between the bortezomib and non-bortezomib groups (p=0.72 and 1.00, respectively). The median PFS in the bortezomib group and the non-bortezomib group was 25.4, and 40.0 months, respectively (p=0.13, Figure 1). The median OS was 61.6 months in the bortezomib group and not reached in the non-bortezomib group (p=0.22, Figure 2). There was no significant impact of high-risk cytogenetics, lactic dehydrogenase (LDH) or b2 microglobulin levels, or maintenance therapy on PFS or OS.
Patient Characteristics And Outcomes
| . | Bortezomib (N=30) N (%) [range] . | No Bortezomib (N=13) N (%) [range] . | P value . |
|---|---|---|---|
| Female | 13 (43) | 7 (54) | 0.74 |
| Median age at auto-HCT | 59.5 [45-75] | 60.5 [47-69] | 0.78 |
| Median interval time from Dx to auto-HCT (months) | 9.0 [4.3-30.9] | 7.8 [3.7-28.1] | 0.59 |
| HR cytogenetics | 6 (25%) | 0 (0) | 0.15 |
| ISS III IIIUnknown | 10 (33) 8 (27) 6 (20) 6 (20) | 4 (31) 4 (31) 3 (23) 2 (15) | 0.97 |
| Bortezomib-based induction | 15 (50) | 6 (46) | 1.00 |
| Median BM plasma cell% at auto-HCT | 3.5 [0-37] | 2 [0-22] | 0.59 |
| Median LDH at auto-HCT | 604 [366-1820] | 510 [362-1114] | 0.35 |
| Median time to neutrophil engraftment (ANC>500) (days) | 10 [8-15] | 10 [9-11] | 0.96 |
| 1-year TRM | 0 | 0 | 1.00 |
| Response post Auto-HCT CRCR + VGPR CR + VGPR + PR | 6 (20) 21 (70) 28 (93) | 7 (59) 10 (77) 13 (100) | 0.03 0.72 1.00 |
| Maintenance | 5 (17) | 4 (30) | 0.41 |
| Median F/U (months) | 61.1 [15.3-80] | 69.5 [27-77.6] | 0.22 |
| Median PFS (months) | 25.4 | 40.0 | 0.13 |
| Median OS (months) | 61.6 | NR | 0.22 |
| . | Bortezomib (N=30) N (%) [range] . | No Bortezomib (N=13) N (%) [range] . | P value . |
|---|---|---|---|
| Female | 13 (43) | 7 (54) | 0.74 |
| Median age at auto-HCT | 59.5 [45-75] | 60.5 [47-69] | 0.78 |
| Median interval time from Dx to auto-HCT (months) | 9.0 [4.3-30.9] | 7.8 [3.7-28.1] | 0.59 |
| HR cytogenetics | 6 (25%) | 0 (0) | 0.15 |
| ISS III IIIUnknown | 10 (33) 8 (27) 6 (20) 6 (20) | 4 (31) 4 (31) 3 (23) 2 (15) | 0.97 |
| Bortezomib-based induction | 15 (50) | 6 (46) | 1.00 |
| Median BM plasma cell% at auto-HCT | 3.5 [0-37] | 2 [0-22] | 0.59 |
| Median LDH at auto-HCT | 604 [366-1820] | 510 [362-1114] | 0.35 |
| Median time to neutrophil engraftment (ANC>500) (days) | 10 [8-15] | 10 [9-11] | 0.96 |
| 1-year TRM | 0 | 0 | 1.00 |
| Response post Auto-HCT CRCR + VGPR CR + VGPR + PR | 6 (20) 21 (70) 28 (93) | 7 (59) 10 (77) 13 (100) | 0.03 0.72 1.00 |
| Maintenance | 5 (17) | 4 (30) | 0.41 |
| Median F/U (months) | 61.1 [15.3-80] | 69.5 [27-77.6] | 0.22 |
| Median PFS (months) | 25.4 | 40.0 | 0.13 |
| Median OS (months) | 61.6 | NR | 0.22 |
HR = high-risk, BM = bone marrow, TRM = transplant related mortality, F/U = follow up
After long-term follow up of 5 years, the addition of bortezomib to a preparative regimen of ATO, AA, and high-dose melphalan did not result in a significant improvement in CR rate, PFS, or OS. The lack of benefit with bortezomib may be due to the inclusion of ATO and AA in the regimen, or the schedule of bortezomib, which was only given before melphalan.
Shah:Celgene: Membership on an entity’s Board of Directors or advisory committees, Research Funding. Qazilbash:Celgene: Membership on an entity’s Board of Directors or advisory committees Other; Millenium: Membership on an entity’s Board of Directors or advisory committees, Membership on an entity’s Board of Directors or advisory committees Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal