Abstract
The combination of tacrolimus/sirolimus (Tacro/Siro) has been evaluated as a promising graft-versus-host disease (GVHD) prophylaxis regimen with reduced acute GVHD rates and decreased non-relapse mortality (NRM) by several groups. However, the Tacro/Siro combination has been associated with increased risks of veno-occlusive disease (VOD) and thrombotic microangiopathy (TMA), which often requires cessation of Tacro, Siro, or both drugs early after HCT. Mycophenolate mofetil (MMF) is one of the standard alternatives to Tacro/Siro in these situations. In this study, we evaluated a cohort of patients who had to switch their planned prophylaxis of Tacro/Siro to an MMF-based alternative to determine their transplant characteristics and outcomes.
Between January 2009 and September 2011, a total of 411 consecutive patients with hematologic disorders underwent allogeneic hematopoietic cell transplantation (HCT) using Tacro/Siro-based GVHD prophylaxis at our institution. Under the IRB-approved protocol, we reviewed their medical records and identified 57 patients (13.9%) who had to discontinue Tacro (n=24), Siro (n=8), or both drugs (n=25) and started MMF within 30 days of transplant. These 57 cases excluded patients who required MMF after having developed GVHD since the study objective was to evaluate the use of MMF as GVHD prophylaxis, not as a treatment for GVHD. In addition to MMF, steroids were used within 30 days of transplant in 77% of these patients (n=44). The patients (median age 57: range 28-73, 21 females and 36 males) received reduced-intensity conditioning (fludarabine/melphalan: n=50) or fully ablative conditioning (FTBI/Cyclophosphamide: n=7) followed by allogeneic HCT from related (n=13) or unrelated (n=44) donors. All patients but two received peripheral blood hematopoietic stem cells. The planned GVHD prophylaxis was Tacro/Siro only (n=45) or Tacro/Siro plus mini-methotrexate (n=9) or r-ATG (n=3). Patient diagnoses included AML (n=11), MDS (n=15), NHL (n=12), ALL (n=6), MPD (n=4), CML (n=3), Hodgkin lymphoma (n=2), or CMMoL (n=1), PLL (n=1). Thirty-seven patients (65%) had clinically significant co-existing disease/organ impairment with a median comorbidity index of 1 (range: 0-6).
The patients required MMF at a median of 10 days post-HCT (range: day -2 to day +30) due to renal dysfunction (n=25), TMA (n=13), confusion (n=8), VOD (n=4), hypertriglycemia (n=2), or other Tacro/Siro-related toxicities (n=5). Serum creatinine level at the time of MMF start was significantly higher at 1.3mg/dl (median range: 0.4-3.0) than at baseline of 0.88 mg/dl (range: 0.5-1.7) (<0.0001). Some of these patients were able to resume Tacro (n=22) and/or Siro (n=13) at later time.
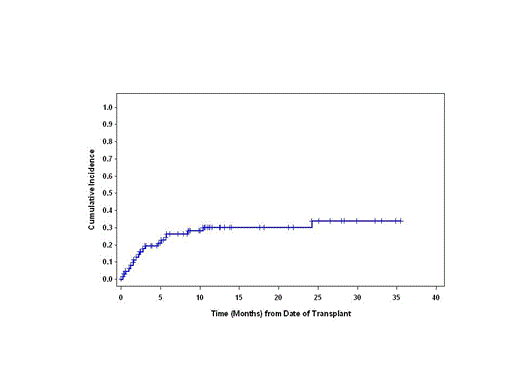
Among these 57 patients, 28 (49%) subsequently developed grade II-IV acute GVHD (grade II: n=14, grade III-IV: N=14). After a median follow up of 15.2 months for surviving patients, the 1-year overall survival probability was 75.8% (66.6-82.8) while 100-day and 1-year non-relapse mortality (NRM) rates were 19.4% (11.7-32.2) and 30.0% (20.3-44.3), respectively (Figure). The causes of deaths include relapse/disease progression in 7, GVHD in 8, infection in 3, graft failure in 2, alveolar hemorrhage in 2 and others in 5.
In summary, we identified a significant proportion of patients (13%) requiring to discontinue Tacro, Siro, or both drugs early post-HCT when the combination of Tacro/Siro was used as GVHD prophylaxis. MMF-based salvage prophylaxis was associated with a relatively good control of GVHD. The NRM and OS rates were also in an acceptable range given the high-risk nature of these patients who experienced significant early toxicities. Further analyses of the current cohort regarding the risk factors for early Tacro/Siro failures will be important in order to optimize patient selection and/or dosing of the combination of Tacro/Siro, both of which have a narrow therapeutic index.
Non-Relapse Mortality (n=57)
Off Label Use: mycophenolate mofitil, sirolimus, and tacrolimus for GVHD prophylaxis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal