Abstract
The prognosis of adult patients (pts) with acute lymphoblastic leukemia (ALL) is dismal, and allogeneic stem cell transplantation (allo-SCT) is performed in most pts. However, even for pts treated with allo-SCT using the standard regimen of cyclophosphamide with total body irradiation (CY/TBI), the prognosis is not satisfactory due to a high rate of relapse. We previously reported excellent outcomes in adult pts with ALL undergoing allo-SCT conditioned with medium-dose VP-16, CY and TBI (medium-dose VP/CY/TBI). We therefore conducted a prospective nationwide multicenter phase II trial (UMIN trial number 000001672) to evaluate the efficacy of this conditioning regimen.
The eligibility criteria of this study were as follows: (1) diagnosis of ALL or acute biphenotypic leukemia (ABL), (2) aged 15-49 years, (3) in CR, (4) first SCT, (5) PS (ECOG) 0-2, (6) intact organ function, and (7) HLA-serologically 6/6 matched donor. Pts with Burkitt leukemia were ineligible for this study. Conditioning regimen consisted of medium-dose VP (15 mg/kg once daily i.v. for two days) and CY (60 mg/kg once daily i.v. for two days) combined with fractionated TBI (total dose: 12 Gy). Stem cell source was limited to bone marrow (BM) or peripheral blood stem cell (PBSC). The primary endpoint of this study was event-free survival (EFS) at one year after SCT, and the events were defined as death or relapse. The expected 1-year EFS was estimated to be 75%, and the threshold 1-year EFS was estimated to be 55%, on the basis of our previous observations.
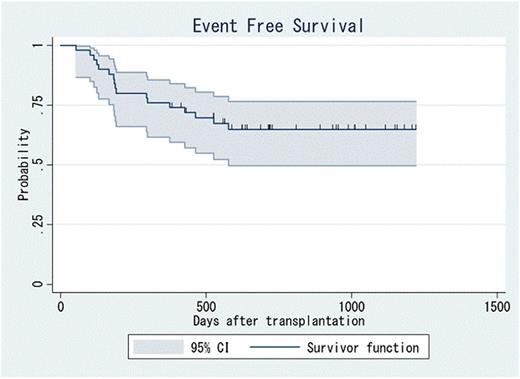
Between February 2009 and August 2011, 52 pts were enrolled, and 50 pts met the criteria. Among 50 eligible pts, the median age was 33.5 years (range, 17-49 years), and 15 pts (30%) were over 40. Twenty-three (46%) pts were female. Forty-eight (96%) pts had ALL and 2 (4%) had ABL. Nineteen (38%) pts were Philadelphia chromosome (Ph)-positive. Forty-seven (94%) pts were in first CR at SCT, and 3 (6%) in second CR. Among pts with ALL in first CR (n=45), 38 (84%) had high-risk disease. Twenty-six (52%) pts underwent SCT from a related donor and 24 (48%) from an unrelated donor. Forty (80%) pts received BM and 10 (20%) received PBSC. All pts achieved neutrophil engraftment. The incidence of grade II-IV and III-IV acute graft-versus-host disease (GVHD) was 38% and 8%, respectively, and that of chronic GVHD was 54%. After 1-year follow-up of the final enrollment (range, 379-1218 days), the 1-year EFS was 76% (95% confidence interval (CI): 62-86%, Figure). One-year overall survival was 80% (95% CI: 66-89%). No pts died within 100 days after SCT and 1-year non-relapse mortality (NRM) was 14% (95% CI: 6-25%). No secondary malignancies were observed during the-follow-up period. The 100-day and 1-year relapse rate was 2% (95% CI: 0-9%) and 10% (95% CI: 4-20%), respectively. The 1-year EFS for pts with high-risk and standard-risk disease was 76% (95% CI: 59-87%) and 71% (95% CI: 26-92%), Ph-positive and negative pts was 79% (95% CI: 53-92%) and 76% (95% CI: 56-88%), and pts over 40 years and under 40 years was 73% (95% CI: 44-89%) and 77% (95% CI: 59-88%), respectively. In univariate analysis, high-risk disease, Ph-positivity and higher age were not significant risk factors for EFS.
The results demonstrate that the conditioning regimen of medium dose VP/CY/TBI for allo-HCT in adult pts with ALL enable good disease control without increase in NRM, even for relatively high age pts and pts with high-risk disease. A phase III trial comparing this regimen with standard CY/TBI regimen for adult pts with ALL is warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal