Abstract
For decades, the standard methods of performing bone marrow and bone biopsy procedures in patients with varying bone densities have remained unchanged; despite the fact that the manual method of performing these biopsies has significant limitations; including patient discomfort, needle-related adverse events, and operator physical ability. As a consequence, interventional radiologists are often asked to perform these procedures for patients with difficult-to-reach biopsy targets. In 2010, a new battery/rotary powered biopsy device was introduced, initially for bone marrow sampling; then for examination of focal lesions of bones; procedures often performed by interventional radiologists using image guidance. In a retrospective study sponsored by Vidacare Corporation, an examination of one radiology and one pathology groups' initial experience using the device at a single center was conducted to determine the performance characteristics of the new biopsy device.
Medical records at Holy Family Hospital from March 2010 to July 2012 were examined, and data for patients who had undergone biopsy procedures using the powered device (OnControl, Vidacare Corp, Shavano Park, TX) were compiled. Data included patient demographics, biopsy type, imaging type, analgesia/anesthesia type, anatomical location, number of passes, room time, procedure time, and complications. Pathology data included specimen dimensions, grading for crush and thermal artifact, presence of hemorrhage, overall quality, and ability to provide a definitive or descriptive diagnosis.
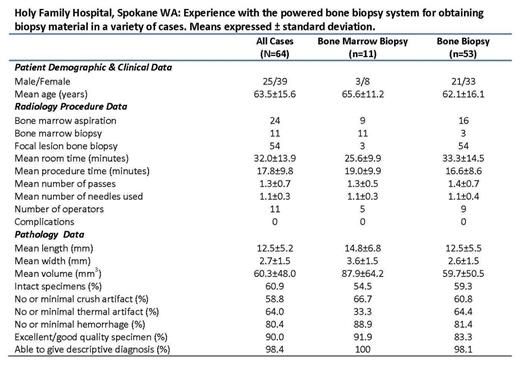
64 patients had biopsy procedures using the powered device by 11 clinicians. Mean patient age was 63.5±15.6; 61% were female. Eleven patients received bone marrow aspiration and biopsy (BMAB) to diagnose/rule out hematological disorders. For the bone marrow biopsy procedures, pathology examination revealed the mean specimen length was 14.8±6.8mm; mean specimen volume was 87.9±64.2mm3 (vs. mean length of 11.0±5.5mm and mean volume of 20.4±9.0mm3 reported for patients receiving manual biopies in a separate study*) Of those specimens, 54.5% were intact, 91.9% were graded excellent or good (compared to 80% rated excellent or good for patients receiving manual biopsies in a separate study*), and the pathologist was able to provide either definitive or descriptive diagnosis for 100% of the cases. For the remaining cases, patients received bone biopsy procedures for focal lesions. Procedures were performed on vertebrae and the ilium in 39% and 38% of the cases, respectively. Other bones included the sacrum, femur, pubis, humerus, rib, and tibia. For all procedures, the mean number of passes was 1.3±0.7, and mean procedure time was 17.8±9.8 minutes. There were no complications.
For random marrow sampling and focal lesions, the powered system yielded outstanding specimens, particularly with respect to volume. Use of the powered device resulted in increased yield rates and higher quality specimens compared to manual devices; as well as easier and faster performance of biopsies, a broader spectrum of potential users (due to the decreased physical requirements for the biopsy), and reduced radiation exposure to patients and operators. The powered devices were especially useful when sampling hard bones and difficult-to-reach bone lesions. Shorter procedure time and diminished physician effort using the new system vastly improved operator ergonomics. The system also transformed previously inaccessible focal lesions into viable biopsy targets.
*Berenson JR, Yellin O, Blumenstein B, Bojanower D, Croopnick J, Aboulafia D, et al. Using a powered bone marrow biopsy system results in shorter procedures, causes less residual pain to adult patients, and yields larger specimens. Diagnostic Pathology 2011;6:23
Symington:Vidacare Corporation: Consultancy, Honoraria, Research Funding. Off Label Use: Not really off-label but could be interpreted as off-label. The study device has a specific indication to be used to biopsy bone marrow, bones of the iliac crest and vertebra and a general indication for bone lesions. Some specific bones other than iliac crest and vertebra were treated, studied and mentioned in this abstract, which could be interpreted as off-label use. Martinez:Vidacare Corporation: Research Funding. Cohen:Vidacare Corporation: Research Funding. Miller:Vidacare Corporation: Co-inventor of study device, Co-inventor of study device Patents & Royalties, Employment. Philbeck:Vidacare Corporation: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal