Abstract
Primary immunoglobulin amyloidosis (AL) is caused by deposition of insoluble amyloidoigenic fibrils of monoclonal light chains, which is often associated with an underlying plasma cell dyscrasia. The heterogeneous clinical manifestations commonly include nephrotic syndrome, hepatic failure, autonomic and sensory neuropathy. Cardiac involvement is most common in immunoglobulin variants of amyloidosis and is associated with significant mortality with survival of 17% by 2 years. Cardiac Magnetic Resonance (CMR) is a non-invasive technique that allows identification of heart involvement in 80% of biopsy proven patients (pts). In systemic amyloidosis with heart involvement, diuretics, despite representing backbone treatment of Congestive Heart Failure (CHF), exacerbation of hypotension and deterioration of cardiac performance are common features.(Dubrey.2012.QJM) Here, we describe a case of a 71 year-old man with subclinical AL amyloidosis who developed severe pulmonary edema during proteosomal inhibition (PI) plus dexamethasone induction.
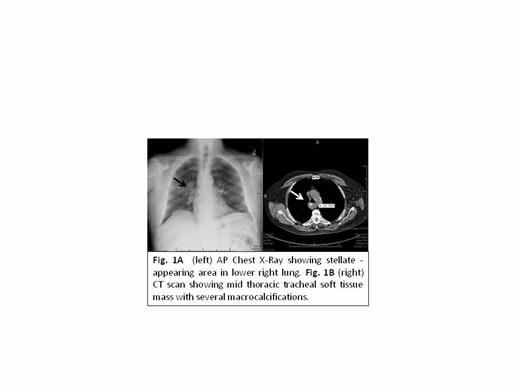
Our pt presented with mediastinal mass (Fig. 1A) detected by the time of pre-operative evaluation for benign prostate hypertrophy surgery. He has a long-term-history of diabetes type 2 metabolically controlled and normal hemoglobin A1C. Further characterization of his mediastinal mass by chest tomography showed a 48 x 26 x 39 mm mass arising from posterior right lateral tracheal wall.
Biopsy sections correlating with kappa light chain AL deposition by mass spectrometry (Fig. 2). Biochemical evidence of elevated kappa free light chain (FLC) and biclonal m-protein revealing IgG and IgA kappa protein by serum protein electrophoresis (SPEP) were observed. His bone marrow aspirate and biopsy was consistent with kappa MGUS (5% clonally restricted plasma cells). Despite no clinical or echocardiographic signs of cardiac dysfunction, left ventricle non-CAD scarring suggesting subclinical amyloid deposition was detected by CMR (Fig. 3). In an attempt to induce hematological response, Bortezomib (B) intravenously (IV) at 1.3 mg/m2 plus weekly oral dexamethasone were initiated. Severe pulmonary edema associated with hemoptysis developed during cycle (C) 1 day (d) 5 of treatment. His repeated chest angiography showed no evidence of pulmonary embolism. A bronchoscopy failed to revealed tracheal perforation. Progressive resolution of symptoms was achieved after administration of furosemide IV. After recovery, pt completed 4 cycles of B plus dexamethasone and proceeded with autologous stem cell transplantation without recurrent pulmonary edema episodes.
Our case suggests potential clinical phenotype associated with subclinical heart amyloid deposition, and propensity for acute pulmonary edema associated with PI recognized by CMR. Although pulmonary edema is uncommonly seen associated with PI in the treatment of plasma cell clonal disorders, clinically manifested acute pulmonary edema could result in high mortality (Hishao et al. Ann Pharmacother.2010). Our pt clinical manifestations could represent phenotypic expression of transiently impaired cardiac function as results of brisk elevation of unfolded serum FLC during PI (Obeng.Blood.2006). Lack of further similar episodes after PI re-exposure suggests low apoptotic threshold of secretory clone.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal