Abstract
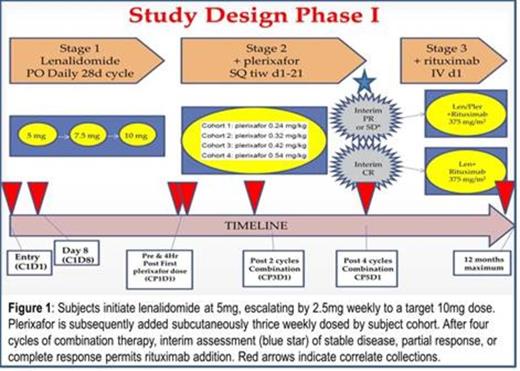
In this single institution phase I clinical trial, subjects were dose escalated on lenalidomide before initiation of plerixafor (Fig. 1). Primary safety response assessment was after one month of combination therapy. After four cycles of combination therapy, the primary response assessment was performed. Peripheral blood was collected for correlates and included an extended CLL phenotype panel (CLL cell-surface markers, CD184/CXCR4, sIgM, CD38, CD40, CD52, CD80, CD86, CD95) by flow cytometry with analysis of CLL subpopulations by CXCR4 expression levels. Phosphoprotein analysis was performed by phosphor-flow cytometry (phosflow).
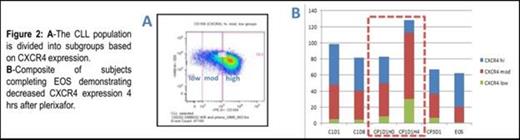
Lenalidomide plus plerixafor is a novel non-cytotoxic therapeutic alternative with an acceptable safety profile in heavily pretreated CLL patients. Grade 3/4 toxicities (and DLTs) were predominantly related to myelosuppression. Though the study is ongoing, the first two patients of cohort 3 have reached safety response assessment without a DLT; therefore a minimum total of 5 subjects were safely treated at dose level one (plerixafor 0.24mg/kg) and it was identified as the MTD. Plans are to proceed to a phase II trial to evaluate efficacy. Early responses among patients able to escalate lenalidomide to 10mg target dose are encouraging given the duration subjects remained on trial without progression. Correlates support mobilization of CLL from the microenvironment with decreases in CXCR4 expression and downstream signaling. Increases of CD40, CD80 and CD86 on lenalidomide monotherapy suggest immunomodulatory actions. Ongoing study will confirm observed trends, and improve understanding of response and resistance mechanisms for translation into optimized treatment strategies.
Brander:Celgene: Research Funding, Research Fund provided to lab but not author Other. Off Label Use: Lenalidomide and Plerixafor are being studied in this Phase I clinical trial but have not been FDA approved for the indication of CLL. Allgood:Celgene: Research Funding. Lanasa:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal