Abstract
Patients with asymptomatic early Rai or Binet stage chronic lymphocytic leukemia (CLL) do not benefit from mono-chemotherapy. Therefore, clinical observation without treatment (watch&wait; W&W) has been the gold standard for the management of these patients. Chemoimmunotherapy with FCR improves the outcome of patients with advanced CLL, but its efficacy in early stage disease has not been investigated. Several clinical and biological variables identify those patients who have a high risk of an aggressive disease course and who might benefit from early interventions. Consequently, this trial was conducted to test the value of FCR treatment in patients with early stage, high-risk CLL.
This report represents the endpoint and safety analysis of a randomized German-French cooperative phase III trial comparing the efficacy of early versus deferred FCR therapy in treatment-naïve Binet stage A CLL patients with a high risk of disease progression. Risk assessment was performed using 4 prognostic markers: Lymphocyte doubling time <12 months, serum thymidine kinase >10 U/L, an unmutated immunoglobulin heavy chain variable region gene (IGHV) status, and presence of unfavorable cytogenetics (del11q, del17p, trisomy 12) by fluorescence-in-situ hybridization. Presence of at least 2 versus less than 2 of these factors defined “high-risk” versus “low-risk” CLL. High-risk CLL patients were further randomized to receive either 6 cycles FCR (HR-FCR) or to be followed by a W&W strategy (HR-W&W). Patients with low-risk CLL were observed only (LR-W&W).
Between 2005 and 2010, a total of 824 patients was enrolled, 423 patients in 69 centers of the German CLL Study Group and 401 patients in 25 centers of the French Cooperative Group on CLL. The diagnosis of CLL needed to be established no longer than 12 months prior to enrollment and patients were required to present with previously untreated stage Binet A CLL at the time of inclusion. Overall, 800 patients (97.1%) were stratified, 201 of them categorized as high-risk CLL (25.1%). There was no significant difference between high-risk patients from the two study groups regarding common baseline characteristics (e.g., age, sex, comorbidity, immunophenotype) and the distribution of risk factors used for stratification.
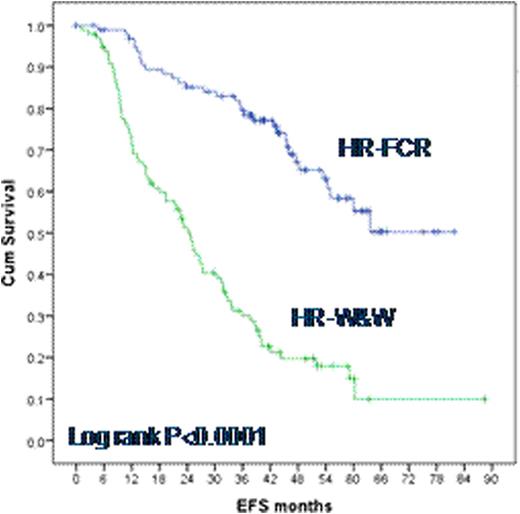
100 out of 201 high-risk patients were randomized to receive FCR therapy (HR-FCR), while 101 patients were allocated to the HR-W&W arm. 18 out of 100 patients (18%) withdrew consent for FCR therapy before treatment was started. 71 (86.6%) of 82 treated patients completed ≥4 cycles. The most common of 228 CTC grade III/IV adverse events reported within 12 months after treatment initiation were hematotoxicity (73.2% of patients) and infections (19.5% of patients). Three patients (3.7%) developed fatal CTC grade V infections (2 septic bacteremias, 1 of them with pulmonary aspergillosis; 1 encephalitis). Out of 79 patients available for response assessment until month 12 after treatment start, 76 showed a complete or partial remission (ORR 96.2%), 2 patients had stable disease (2.5%) and 1 patient had progressed (1.3%). After a median follow up of 46 months (range 0-88 months), HR-FCR patients demonstrated a significantly improved event-free survival (EFS) compared to HR-W&W patients (median EFS not reached versus 24.5 months, respectively, P<0.0001, Fig. 1). Overall survival was not significantly different between HR-FCR and HR-W&W with 181 high-risk patients (90%) being alive at last follow up. Both, HR-FCR and HR-W&W patients exhibited a significant shorter event-free and overall survival than LR-W&W patients, demonstrating an efficient prognostic segregation of patients by the risk assessment used for this trial (analysis based on the German LR-W&W cohort only, complete German-French LR-W&W data will be presented at the meeting).
Kaplan-Meier survival functions for EFS in 100 HR-FCR versus 101 HR-W&W patients.
This is the first randomized phase III trial investigating the efficacy of FCR chemoimmunotherapy in early stage CLL. So far, the study has revealed two major results: 1. A combination of clinical and biological factors can be used to identify early stage CLL patients who experience a rapid disease progression with unfavorable outcome, 2. FCR chemoimmunotherapy substantially improves event-free survival in early stage high-risk CLL.
Langerbeins:Roche: travel grants Other. Cazin:roche: meeting invitation Other, Membership on an entity’s Board of Directors or advisory committees; GSK: meeting invitation, meeting invitation Other, Membership on an entity’s Board of Directors or advisory committees. Fischer:Mundipharma: Travel grants, Travel grants Other; Roche: Travel grants Other. Stilgenbauer:Roche: Consultancy, Research Funding, Travel grants Other; Mundipharma: Consultancy, Research Funding. Leblond:Roche : Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Mundipharma: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau. Hallek:F. Hoffmann-La Roche: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal