Abstract
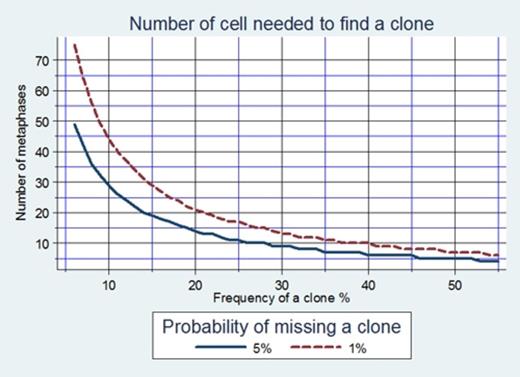
Cytogenetic analysis in myelodysplastic syndromes (MDS) is the “gold standard” method for identification of clonal chromosomal abnormalities, dependent on culturing the bone marrow cells and analyzing at least 20 metaphases. According to the International System for Human Cytogenetic Nomenclature (ISCN 2009) definition of a clone requires at least two metaphases with the same structural abnormality or the same extra chromosome, or alternatively, the presence of three or more metaphases missing the same chromosome. The minimum number of metaphases to detect a clone is calculated according to the formula presented in Fig.1. To avoid missing a clone present in 14% of cells, 20 metaphases must be analyzed, and to avoid missing a clone present in only 10% of cell 30 metaphases must be analyzed, with 95% confidence. Fluorescence in situ hybridization (FISH) on cultured cells is used as a complementary test, permitting analysis of large numbers of cells. The protocol in our lab includes analyzing, independently, both cytogenetic and FISH, on uncultured BM cells.
The aim of our study is to determine the minimal amount of mitoses that must be analyzed by cytogenetic combined with FISH to avoid false-negative results thus optimizing the diagnosis process of MDS patients.
Retrospective study was performed on cytogenetic and FISH results of a group of 325 suspected MDS patients analyzed at the Chaim Sheba Medical Center in the years 2009-2011.
FISH was normal, for the most common MDS abnormalities, (-5,del(5q); -7,del(7q); +8; del(20q), as well as -Y for male patients), in 258 (79.4%) of patients 95.3% of them with normal cytogenetic. One or two abnormal clones, from the intermediate risk group in IPSS, were found in 10 patients (3.9%), at least in 30% of the mitoses. Complex karyotypes with 3 abnormalities, defined as poor risk, were found in two (0.8%) patients in 87.5% of the mitoses. Importantly, when FISH is normal, 9 metaphases are enough to detect minimal size of an abnormal clone with 95 % confidence and 14 metaphases with 99% confidence (Fig.1). Abnormal FISH was detected in 67 of patients. The same abnormalities were found by cytogenetic in 42 (62.6%), at least in 20%of the mitoses. In 25 patients, cytogenetic yielded additional chromosomal aberrations, not detected by FISH, at least in 20% of the mitoses, of them 20 patients (29.9%) with more than one aberration. Notably, in patients with abnormal FISH, analyses of 14 metaphases are enough to detect minimal size of CCA clone present in 20% of mitoses with 95 % confidence and 21 metaphases with 99% confidence (Fig.1). In addition cryptic deletions were detected by FISH, and not by cytogenetic, in 5 patients: del(7q) (n=3),del(5q) and del(20q), one each. Furthermore in 17 of 20 patients (85%) with complex karyotypes del(5q) was involved. Finally, in patients in whom -Y was detected by FISH, cytogenetic did not detect any other chromosomal abnormality implying to the benign nature of this lesion.
The combination of FISH, for the five most common abnormalities, and cytogenetic may improve the accuracy and speed of the diagnostic process, and may permit significant reduction of the number of mitoses to be analyzed in suspected MDS patients. The minimal size of mitoses to be analyzed to detect an abnormal clone has to be defined in each laboratory. We suggest that search for cryptic del(5q), del(7q) and del(20q) by FISH be added to the routine BM assessment in MDS patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal