Abstract
Chronic myeloid leukemia (CML) accounts for 10-15% of all adult leukemias in the US. CML treatment has improved in the past few years with the introduction of imatinib in 2001, which has improved overall survival in patients with CML. Despite its success, there remains a subset of individuals who are either imatinib-resistant or imatinib-intolerant. With the approval of 2nd generation TKIs (dasatinib, nilotinib) beginning in 2006, approximately 40–50% of imatinib-resistant and imatinib-intolerant patients achieve complete cytogenetic response (CCyR) when switching TKI. Few studies have estimated duration of TKI use since the approval of 2nd generation TKIs. The objective of this study is to explore current treatment patterns of TKIs by line of therapy in the US.
U.S. health plan claims data from 01 January 2007 through 31 December 2011 were used to examine duration of TKI treatment by line of therapy for patients with CML aged 18 and older. Patients were required to have a prescription fill for a TKI and a CML diagnosis (ICD-9-CM code 205.1). Duration of TKI use was determined based on a gap in TKI coverage of 180 or more consecutive days following TKI initiation or switch to another TKI within the 180 day window. To account for censoring due to disenrollment for the health plan or end of the study period, median duration of 1st and 2nd line treatment is estimated via the Kaplan-Meier estimator.
We identified 695 who started TKI treatment and had diagnosis for CML from 2007 through 2011. More than half (58%) were male; mean age was 55 years (SD 14 years). The most common first line TKI was imatinib (82%) with dasatinib and nilotinib use equally distributed (9%). Of patients starting a 2nd line TKI, dasatinib represented 59% and nilotinib 37% (Table).
Baseline Characteristics: Patients with CML
| . | 1st TKI . | 2nd TKI . |
|---|---|---|
| N | 695 | 148 |
| Age, years, mean (SD) | 55 (14) | 54 (14) |
| Male sex, n (%) | 405 (58) | 78 (53) |
| TKI | ||
| Imatinib, n (%) | 571 (82) | 6 (4) |
| Dasatinib, n (%) | 65 (9) | 87 (59) |
| Nilotinib, n (%) | 59 (9) | 55 (37) |
| Time to switch, mos, mean (SD) | -- | 12 (10) |
| . | 1st TKI . | 2nd TKI . |
|---|---|---|
| N | 695 | 148 |
| Age, years, mean (SD) | 55 (14) | 54 (14) |
| Male sex, n (%) | 405 (58) | 78 (53) |
| TKI | ||
| Imatinib, n (%) | 571 (82) | 6 (4) |
| Dasatinib, n (%) | 65 (9) | 87 (59) |
| Nilotinib, n (%) | 59 (9) | 55 (37) |
| Time to switch, mos, mean (SD) | -- | 12 (10) |
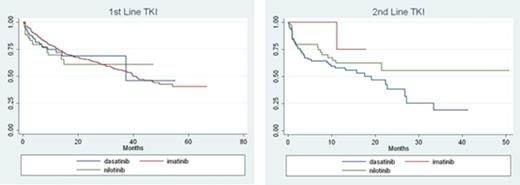
Since 2007, the median duration of 1st line TKI was 39 months and 2nd line TKI was 22 months. Median duration of treatment for 1st line (p=0.4342) and 2nd line treatment (p=0.1762) were not significantly different by TKI (Figure). Among those who initiated 2nd line TKI, the majority were switched from imatinib to dasatinib or nilotinib (86%). Mean time to switch was 12 months and did not differ when switching from imatinib to dasatinib or nilotinib (p=0.7233).
Duration of TKI therapy is an informative measure as it is a proxy measure of treatment effectiveness as well as patient adherence, which is important to maintain optimal therapeutic outcomes. Among CML patients, we found median duration of treatment in both the 1st and 2nd line to be similar by TKI.
Henk:OptumInsight: Employment. Woloj:Pfizer Inc.: Employment. Shapiro:Pfizer Inc.: Employment. Whiteley:Pfizer Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal