Abstract
Recent studies suggest that aberrant methylation plays a fundamental role in the development of a variety of cancers, including myeloid malignancies. Juvenile myelomonocytic leukemia (JMML) is an aggressive myeloid neoplasm of early childhood that is characterized by both excessive proliferation of myelomonocytic cells and hypersensitivity to granulocyte-macrophage colony-stimulating factor. It is categorized as an overlap myelodysplastic syndrome/myeloproliferative neoplasm (MDS/MPN) according to the World Health Organization classification. We recently reported that somatic mutations in SETBP1 and JAK3 were identified in JMML patients and were associated with poor outcomes (Nat Genet 2013;45:937–41). The goal of this study was to clarify the clinical significance of aberrant DNA methylation in JMML.
We studied 92 children (61 boys and 31 girls) who were diagnosed with JMML in institutions throughout Japan. A diagnosis of JMML was made based on internationally accepted criteria. We quantitatively evaluated the CpG methylation pattern in the promoter regions of 16 candidate genes (APC, BMP4, CALCA, CDH13, CDKN2A, CDKN2B, CHFR, DAPK, DMR-H19, ER, IGF2, MGMT, MLH1, RARB, RASSF1, TP73) from genomic DNA derived from bone marrow specimens at the time of diagnosis. This was accomplished by bisulfite conversion and the pryosequencing technique. We defined aberrant methylation as >3 standard deviations from the mean methylation level derived from 8 healthy individuals. The median age at diagnosis was 16 months (range, 0.3–160). By genetic analysis, PTPN11, NF1, NRAS, KRAS, and CBL mutations were found in 39 (42%), 7 (8%), 12 (13%), 13 (14%), and 11 (12%) patients, respectively. In addition, 16 patients had SETBP1 or JAK3 mutations. Karyotypic abnormalities were detected in 15 patients, including 8 with monosomy 7. The median monocyte count, percentage of hemoglobin F, and platelet count at the time of diagnosis were 4.6x109/L (range, 0.2–31.6), 21% (range, 0–68), and 61.0x109/L (range, 1.4–483), respectively. The median observation period was 18 months (range, 1–287). During observation, 56 of the 92 patients received allogeneic hematopoietic stem cell transplantation (HSCT), and 30 of 92 patients died. Outcomes were assessed according to transplantation-free survival (TFS), in which HSCT and death were censored, and overall survival (OS) by the Kaplan-Meier method.
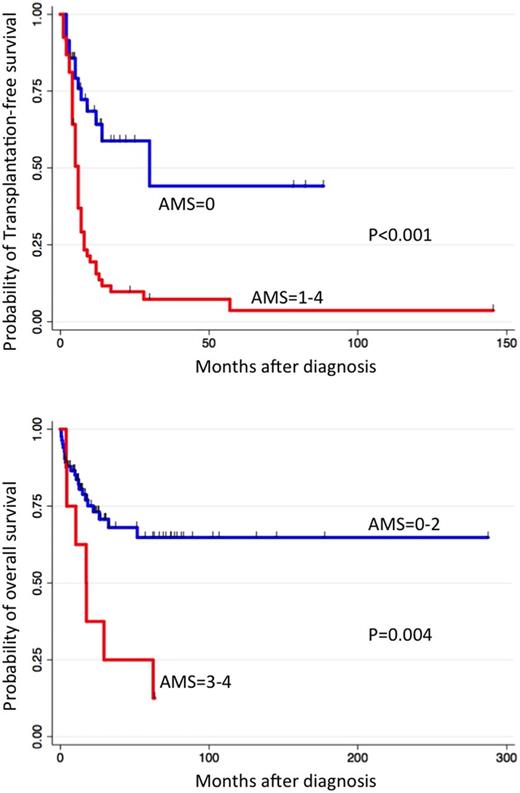
Aberrant methylation of BMP4, CALCA, CDKN2A, CDKN2B, DMR-H19 and RARB were detected, of which hypermethylation of BMP4, CALCA, CDKN2A, and RARB were associated with poor TFS according to univariate analyses (P<0.10). We integrated the number of aberrant methylation of these four genes to arrive at an aberrant methylation score (AMS). An AMS of 0, 1, 2, 3, and 4 was seen in 36, 29, 19, 7, and 1 of the 92 patients, respectively. The AMS was significantly higher in patients with SETBP1 or JAK3 mutations than in other patients (P=0.03): 1, 8, 3, 3, and 1 of the 16 patients showed an AMS of 0, 1, 2, 3, and 4, respectively. The probability of 5-year TFS was 42% in the AMS = 0 cohort and 4% in the AMS = 1 to 4 cohort, respectively (log-rank, P<0.001). Moreover, the probability of 5-year OS was 65% in the AMS = 0 to 2 cohort and 8% in the AMS = 3 and 4 cohort, respectively (log-rank, P=0.004). In multivariable analysis using the Cox-proportional hazard model, AMS = 1 to 4 (hazard ratio [HR], 2.6; 95% confidential interval [CI], 1.2–5.5; P=0.013), mutations of PTPN11 or NF1 (HR, 2.7; 95% CI, 1.3–5.5; P=0.010), and chromosomal aberration (HR, 3.3; 95% CI, 1.7–6.5; P=0.001) were independent predictors of poor TFS. Further, chromosomal aberration (HR, 4.4; 95% CI, 1.6–11.8; P=0.004) and platelet counts <33x109/L (HR, 2.8; 95% CI, 1.3–6.4; P=0.013) were independent predictors of poor OS.
Makishima:AA & MDS international foundation: Research Funding; Scott Hamilton CARES grant: Research Funding. Maciejewski:NIH: Research Funding; Aplastic anemia&MDS International Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal