Abstract
NPM1 mutations (NPM1mut) are known to be prognostically favorable in the absence of FLT3-ITD. About 80% of patients (pts) have previously been shown to carry a distinct NPM1mut (“type A”). The remaining NPM1mut pts show various rare mutations (“rare types”) with different individual frequencies. Whether these “rare types” differ from “type A” with respect to clinical outcome and accompanying genetic abnormalities is unknown.
To evaluate the incidence of “rare types” of NPM1mut in AML and to investigate whether they show differences in cytogenetics, concomitant molecular mutations, biological or prognostic profiles compared to pts with the common “type A”.
A cohort of 2,859 newly diagnosed adult AML pts were screened for NPM1mut by melting curve analysis and subsequent Sanger sequencing of the positive cases. In 877 (30.7%) pts NPM1mut were detected. All 877 NPM1mut pts were also analyzed for FLT3-ITD. The following markers were screened in the respective subcohorts: FLT3-TKD (n=869), MLL-PTD (n=872), WT1 (n=785), CEBPA (n=690), IDH2R172 (n=590), IDH1R132 (n=582), IDH2R140 (n=577), RUNX1 (n=531), DNMT3A (n=529), ASXL1 (n=432), NRAS (n=390), KRAS (n=231), and TET2 (n=216).
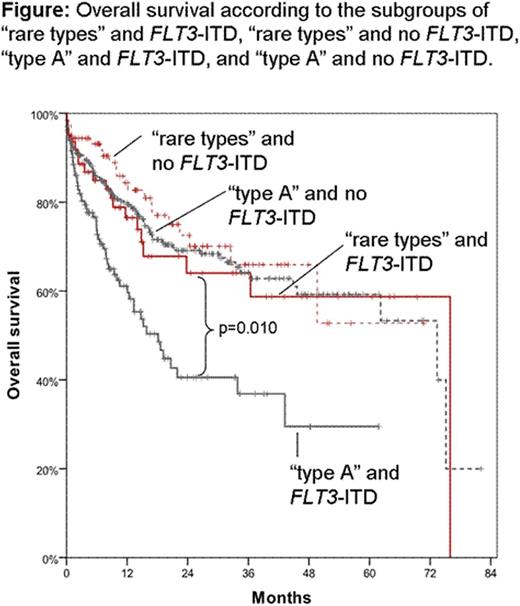
In total, 660/877 (75.3%) mutations were “type A” mutations (c.860_863dupTCTG), whereas 217/877 (24.7%) represented “rare types”. The most common of the latter was type B (c.863_864insCATG; n=77; 8.8% of all NPM1mut) followed by type D (c.863_864insCCTG; n=56; 6.4%), and type I (c.863_864insCTTG; n=15; 1.7%). The remaining 41 types were present in less than 1% each. Pts with “rare types” were slightly younger than pts with “type A” (mean 61.4 yrs vs 63.8 yrs; p=0.035) and showed a trend for lower WBC counts (50.6 vs 61.2x109/L; p=0.051). Neither gender, hemoglobin level, platelet count, the history of the disease, the percentage of bone marrow blasts, normal vs aberrant karyotypes, nor distribution between the intermediate and adverse cytogenetic risk scores according to the refined MRC criteria varied between “rare types” and “type A”. “Rare types” more often harbored WT1 mutations than “type A” pts (10.6% vs 3.3%; p<0.001) and less frequently had DNMT3A mutations (33.0% vs 58.0%; p<0.001). No associations were seen for any of the other molecular mutations. Focusing on FLT3-ITD, no differences within the FLT3-ITD/wt ratio was seen between “rare types” and “type A”. Focusing on the prognostic relevance, we limited our cohort to 603 intermediate-risk karyotypes pts with available information on outcome who have been treated in curative intention: “Rare types” (n=153) had a trend towards a favorable overall survival (OS) compared to “type A” (n=450; median 76.0 vs 62.2 months (m); p=0.083). Same holds true for event-free survival (EFS) (20.3 vs 14.7 m; p=0.084). Restricting the analysis to pts with FLT3-ITD, “rare types” (n=63) showed significantly better OS than “type A” pts (n=182; 76.0 vs 20.7 m; p=0.049) and as well better EFS (22.8 vs 8.0 m; p=0.021). Even more striking: dividing the cohort to the 4 possible combinations of these two markers we found 1) 63 pts presenting with “rare types” and FLT3-ITD, 2) 90 with “rare types” and no FLT3-ITD, 3) 131 pts with “type A” and FLT3-ITD, and 4) 319 pts with “type A” and no FLT3-ITD. Only those pts harboring “type A” and FLT3-ITD showed adverse OS (18.1 m) compared to all other subgroups (p=0.010 vs “rare type” and FLT3-ITD; p<0.001 vs. the two other subgroups). Furthermore, the three other subgroups did not differ amongst each other in OS (“rare type” and FLT3-ITD: 76.0 m; “rare type” and no FLT3-ITD: not reached; “type A” and no FLT3-ITD: 73.4 m; n.s.; Figure). This effect is also seen for EFS where “type A” and FLT3-ITD pts showed adverse EFS (6.9 m) compared to all other subgroups (p=0.006 vs “rare types” and FLT3-ITD; p<0.001 vs. the two other subgroups). Again, the three other subgroups did not differ amongst each other (“rare types” and FLT3-ITD: 22.8 m; “rare types” and no FLT3-ITD: 18.1 m; “type A” and no FLT3-ITD: 18.7 m; n.s.).
1) Rare NPM1mut types harbored more frequently WT1mut and less frequently DNMT3Amut. 2) The adverse effect of FLT3-ITD mutations within NPM1mut pts is not seen within the “rare types”, but only in those pts showing NPM1mut “type A”. 3) Therefore, NPM1mut pts showing “rare types” might not be stratified by their FLT3-ITD status, but by their prognostically favorable NPM1mut only.
Alpermann:MLL Munich Leukemia Laboratory: Employment. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Dicker:MLL Munich Leukemia Laboratory: Employment. Eder:MLL Munich Leukemia Laboratory: Employment. Kohlmann:MLL Munich Leukemia Laboratory: Employment. Kern:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Schnittger:MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal