Abstract
The intergroup PRIMA Phase III study was designed to investigate the potential benefit of 2-years of rituximab maintenance in patients with follicular lymphoma (FL) responding to one of three non-randomised first line immunochemotherapy treatments. The results of the final analysis with 36 months follow-up (Salles et al., Lancet 2011) demonstrated a significant reduction of the risk of progression or death with a hazard ratio (HR) of 0.55 in favour of patients randomized to rituximab maintenance. We present here the updated results with 3 additional years of follow-up.
From December 2004 until April 2007, 1217 patients were enrolled from 223 centres and complete data were available for 1193 patients who had the following pre-induction treatment characteristics: median age 56 years [range 22–87]; 52% male; 90% Ann Arbor stage III-IV; 33% B symptoms; 56% bone marrow involvement; 4% ECOG performance status >1; 34% elevated LDH; 32% β2-microglobulin >3mg/L; FLIPI score 0-1 (21%), FLIPI 2 (36%), FLIPI 3-5 (43%). Most patients (75%) received R-CHOP induction (22% R-CVP, 3% R-FCM). Patients responding to induction therapy were stratified based on their immunochemotherapy regimen and response [CR/CRu versus PR] and randomized to observation or rituximab maintenance, 1 infusion (375 mg/m2) every 8 weeks for 2 years. A total of 1018 randomised patients were analyzed according to the ITT principle (513 observation / 505 rituximab maintenance). All initial pre-treatment characteristics were well balanced between arms and the response status at time of randomization was CR=39%; CRu=32% and PR=28% (others 1%).
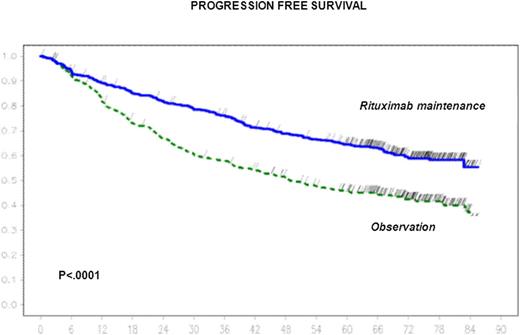
With a median follow-up of 73 months from randomization, 6-year progression free survival estimate was 42.7% (95% CI 38 – 46.9%) in the observation arm (284 events, median=48 months) and 59.2% (95% CI 54.7 – 63.7%) in the rituximab maintenance arm (194 events, median not reached), respectively (stratified Log-Rank, P<. 0001; HR = 0.58 ; 95% CI 0.48 - 0.69).
In pre-planned analyses of patients subgroups categorized by age, sex, FLIPI score category, induction chemotherapy and response to induction, the effect of rituximab maintenance was examined and found to be consistent among these different subgroups. In a Cox regression multivariate analysis, rituximab maintenance (HR=0.57; P<.0001) as well as older age (HR=0.79; P=.015), female sex (HR=0.72; P=.0003) and low or intermediate FLIPI groups (HR=0.67; P<.0001) were all significant variables associated with superior progression free survival. A significant reduction in the risk of starting a new anti-lymphoma treatment (HR=0.63, 95% CI 0.52 - 0.76) or starting a new chemotherapy (HR=0.70, 95% CI 0.57 - 0.86) were also observed for rituximab maintenance.
The rate of histological transformation did not appear to differ between the 2 treatment arms: in the observation arm, transformation was documented in 24 patients (114 cases with morphological documentation out of 278 progressions) versus 16 patients in the rituximab maintenance arm (80 out of 186) respectively. Overall response rate to second-line therapy was reported by investigators to be 180/227 (79%) in patients from the observation arm (CR/CRu=61%; PR=19%) versus 109/144 (76%) in patients from the rituximab maintenance arm (CR/CRu =51%; PR=22%) (P=NS).
At the time of the data cut-off, overall survival (OS) remains favourable in both study arms: 58 patients (11.3%) have died in the observation arm (6-years OS estimate 88.7%) compared to 59 patients (11.7%) in the rituximab maintenance arm (6-year OS estimate 87,4%). Main causes documented for death in the observation and rituximab maintenance arm respectively were lymphoma (28 ; 28), other malignancy (19 ; 5) and infections (4 ; 7). No new significant safety data were captured with this additional follow-up period.
Salles:Roche: Consultancy, Honoraria, Research Funding. Seymour:Roche: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau, Travel support Other; Genetech: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Feugier:Roche: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Offner:Lilly: Membership on an entity’s Board of Directors or advisory committees. Lopez-Guillermo:Roche: Membership on an entity’s Board of Directors or advisory committees. Belada:Roche: Consultancy. Catalano:Roche: Membership on an entity’s Board of Directors or advisory committees. Haioun:Roche: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Simpson:Janssen Research & Development: Honoraria. Leppa:Roche: Consultancy, Honoraria, Research Funding, Travel support Other. Soubeyran:Roche: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Hagenbeek:Takeda/Millennium: Consultancy. Casasnovas:ROCHE: Consultancy, Honoraria, Research Funding. Coiffier:Millennium Pharmaceuticals : Consultancy. Tilly:Roche: Honoraria; Celgene: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity’s Board of Directors or advisory committees; Pfizer: Honoraria; Janssen: Honoraria; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal