Abstract
Green tea (GT) is chemically characterized by the presence of large amounts of polyphenolic compounds. The beneficial effects of green tea are well known. A number of epidemiologic studies have linked consumption of green tea to decreased risk of cancer, in vitro and animal models have supported green tea’s ability to inhibit the different stages of cancer development. Acute myeloid leukemia (AML) is an aggressive hematologic malignancy. The development and progression of the leukemic disease involve deregulation of cell death response resulting in failure of production of normal blood cells. Accordingly, the goal of this work was to identify the effects of oral green tea administration in a xenografted model of leukemia as well as in a 65-year old patient diagnosed with AML with myelodysplasia-related changes.
In vivo: HL-60 cells were maintained in 10% of RPMI medium. The HL-60 xenotransplant was performed using immunodeficient mice (NOD.CB17-Prkdcscid/J lineage (Jackson Lab. USA), (n= 6). Mice were inoculated, subcutaneously, in the dorsal region, 1x107cells/mice. Every 7 days the tumor volumes were evaluated according to the following formula: tumor volume (mm3) = (length x width2)/2. The GT (Galena Pharmaceutical) treatment started after tumors reached 100 to 200 mm3, it was given every day by gavage at 100mg/Kg body weight (diluent: water solution). Control group received equal amounts vehicle solution. After 21 days, the mice were sacrificed, tumors were removed, minced and homogenized in protein extraction buffer or fixed in formalin immediately for immunohistochemistry with antibodies for detection of apoptosis, autophagy and cell cycle processes. Clinical protocol: We performed a pilot study with an elderly AML patient unfit to conventional chemotherapy due to comorbidities and personal desire. Our primary goal was to confirm the suppressive activity of GT against leukemic blasts. Our secondary objective was to analyse possible changes in her adaptive and innate immune system induced by GT. Capsules containing 500 mg of GT were supplied by the Galena pharmaceutical. The patient received 1 capsule (500mg) once per day during 3 months and was evaluated once every 4 weeks by physical examination and laboratory testing.
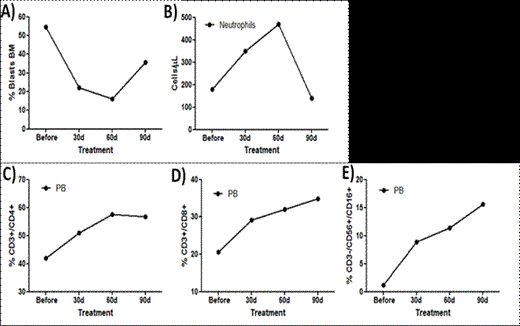
In vivo: A 42% reduction of the tumor volume was observed in mice treated for 21 days with GT treatment, compared to untreated animals. The main proteins related to the autophagy process were analyzed and we found increased expression of beclin-1 and class III PI3K, ATG5-ATG12 and ATG7. Immunohistochemistry analysis showed higher LC3I/II staining in mice treated with GT compared to control. Decreased Bcl2 expression, increased Bax expression and no modulation of BcL-xL levels was also detected by immunohistochemistry in treated animals. Caspase 3 staining was active in GT mice. GT induces cell cycle arrest in S phase, with reduction of cyclin A and CDk2 expression and increased p21 staining. We could not observe any significant difference in cyclin D, cyclin E, Cdk2, 4 and 6 expression. GT promoted pronounced phosphorylation of AKT, ERK1/2 and JNK. Clinical protocol: During GT treatment, the patient did not present any complication, including infections and bleeding. She remained as outpatient during all protocol period.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal