Abstract
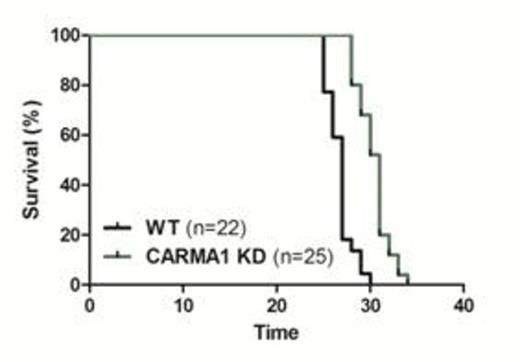
Acute lymphoblastic leukemia (ALL) is one of the most commonly seen childhood lymphomas, and can arise from the T or B cell lineage (T-ALL or B-ALL respectively). T-ALL patients have almost 80% cure rate, however relapse in central nervous system (CNS) remains a major therapeutic challenge. Recently it has been shown that a chemokine receptor CCR7 is an essential regulator of T-ALL cell infiltration into the CNS. CCR7 is a homeostatic chemokine receptor that controls the homing of cells to lymph nodes, including naïve T cells, dendritic cells, and lymphoma cells. The ligands for CCR7, CCL19 and CCL21, are expressed by stromal cells in the secondary lymphoid organs. CCL21 and CCL19 function as chemoattractants for T and B lymphocytes expressing CCR7. Defects in either CCR7 or its ligands impairs lymphocyte migration. Aberrant CCR7 expression has been associated with certain cancers such as gastric cancers, breast cancers and head and neck cancers and been linked to pro-survival and invasive pathways. While CCR7 controls the migration of naïve T cells and T-ALL leukemic cells, little is known about the signaling pathways downstream of CCR7. Numerous studies have demonstrated the importance of Protein Kinase C θ (PKCθ) in normal T cell function as well as T-ALL. PKCθ regulates NF-κB by interaction with a complex of proteins including CARMA1, BCL10, and MALT1, which all function together to activate NF-κB upon T cell receptor ligation. In addition to being crucial for normal T cell activation, PKCθ has also been shown to be important in T-ALL induction in a mouse model of T-ALL. While PKCθ is clearly important in multiple aspects of T cell function, nothing is known about the potential role of PKCθ in controlling migration of T cells, including leukemic cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal