Abstract
Treatment related toxicity complicates outcome in elderly patients with AML (Estey et al. Blood. 2006). Conventionally, 7+3 induction (anthracycline plus cytarabine) results in Complete Remission rate of about 30%. Regimens with less toxicity, such as 10-days (d) schedule of DAC, seem promising with CR rate of 47% (Blum et al. PNAS. 2010). In secondary MDS derived AML, response prediction could be derived from mutation status in epigenetic modifiers (IDH1, IDH2, DNMT3 A, TET2), transcriptional regulators (RUNX1, CBL), and genes in spliceosome machinery, such as SF3B1 and SRSF2 (Husseinzadeh et al. American Society of Hem Meeting. Abstract # 1698. 2012). IDH-1 mutation is known to induce hypermethylator phenotype (fig 1) (Figueroa et al. Cancer Cell. 2010) and might be present in conjunction with SRSF2 mutations, an unique unreported molecular subset, feasible for exploring azanucleoside response prediction. Herein, we report a case of trisomy 8 MDS derived IDH1 and SRSF2 mutated AML who underwent rapid morphological blast differentiation in the context of acute differentiation syndrome while treated with 10 days (d) of hypomethylating dose of DAC.
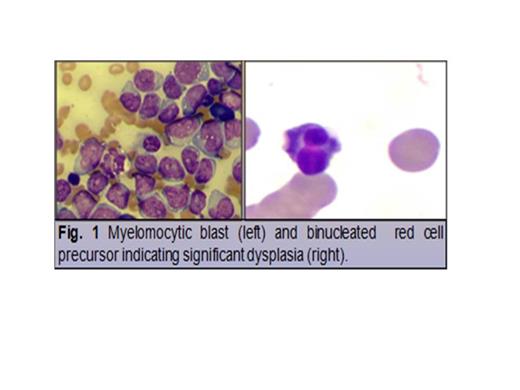
A 61-year-old male with performance status (PS) = 3 presented with leukocytosis of 18.9 K/uL (peripheral blast 90%). He had a history of low-grade MDS diagnosed 2 years before AML transformation. After morphological confirmation of M5 AML, fluorescent in situ hybridation (FISH) revealed 81% nuclei with trisomy 8. Extracted DNA was tested with a custom-designed Leukemia Cancer Gene Mutation Panel using AmpliSeq™ technology and showed IDH1 c.394C>T(p.R132C) mutation (Fig. 2B) and c.284C>T(p.P95L) mutation of SRSF2 gene (Fig. 2C). DAC was initiated at 15 mg/m2 for a total of 10 days every 28 d cycle.
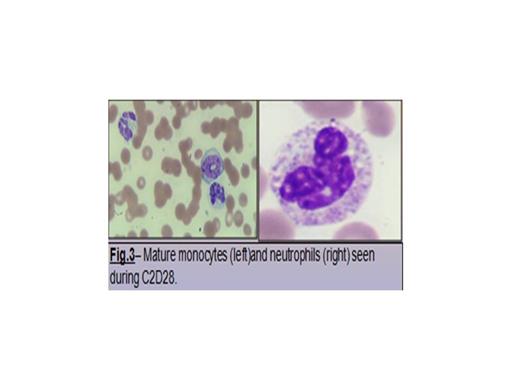
By day 5 of cycle (C)1 of DAC treatment, brisk and significant rebound leukocytosis of 60 K/uL (Fig. 3) was observed, along with shortness of breath, hypoxemia and radiological evidence of floppy bilateral pulmonary infiltrates suggestive of acute-like differentiation syndrome. In addition to broad-spectrum antimicrobial and antifungal, dexamethasone at 4 mg intravenously (IV) every 8-hour (h) and hydroxyurea at 1 g orally every 8 h resulted in progressive normalization of peripheral blood count and hypoxemia after 48 h. Patient (pt) recovered from C1 and proceeded with C2 of treatment. A similar episode of brisk/robust leukocytosis was observed by day 5 of C2 requiring dexamethasone and hydroxyurea. Progressive morphological differentiation was observed to full mature and morphologically normal monocytes and neutrophil (Fig. 4). Pt expired as result of severe clostridium difficile colitis during C3 of DAC.
In our case, we observed robust acute differentiation syndrome characterized by rapid increase of WBC, shortness of breath and hypoxemia associated with azanucleoside treatment. Beside a novel association of IDH-1 and SRSF2 mutations, acute differentiation might suggest potential feature for azanucleoside response phenotype. Our case adds body of evidence of connection between epigenetic regulator and spliceosome mutations. Further studies on the impact of dual mutations in epigenetic reprogramming, leukemia transformation, and azanucleoside response will allow improved decision algorithm and therapeutic design.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal