Abstract
In acute myeloid leukemia (AML) molecular mutations are becoming increasingly important as markers for classification, risk stratification and disease monitoring. Frequencies and prognostic impact of most of the currently known mutations have been widely studied. In contrast, the stability during disease evolution and the role of single markers at relapse are less clear.
1) To compare the patterns of molecular mutations between diagnosis and relapse. 2) To analyze the impact of single mutations on time to relapse (TTR).
We investigated paired diagnostic and relapse samples in a cohort of 556 adult AML cases that were selected based on confirmed relapse with available samples from both time points (483 de novo, 33 t-AML, 40 s-AML). The cohort comprized 255 females and 301 males; median age: 63.0 years (range: 18.6-85.2 years). In total, 5,726 paired analyses were performed (mean: 10.3/pt, range: 2-21). Besides analyses for fusion genes (n=114) the following genes were analyzed in paired samples for mutations in respective numbers: ASXL1: n=464, CBL: n=99, CEBPA: n=374, DNMT3A: n=244, FLT3-ITD: n=534, FLT3-TKD: n=461, IDH1: n=469, IDH2: n=418, KIT: n=34, KRAS: n=49, MLL-PTD: n=165, NPM1: n=343, NRAS: n=105, RUNX1: n=356, TET2: n=243, WT1: n=153, others: n=1,101. Mutations were analyzed by amplicon deep-sequencing, direct Sanger sequencing, gene scan, conventional PCR, quantitative real time PCR or melting curve analyses. In addition, chromosome banding analysis data was available in 552 cases.
629 relapses were detected in the 556 patients (pts). 67 pts had a second and 6 even a third relapse. At diagnosis the subtypes according to cytogenetics were as follows: PML-RARA (n=10), RUNX1-RUNX1T1 (n=24), CBFB-MYH11 (n=23), MLL-translocations (n=30), DEK-CAN (n=3), other recurrent translocations (n=14), complex karyotype (n=30), other aberrations (n=107), normal karyotype (NK, n=311).
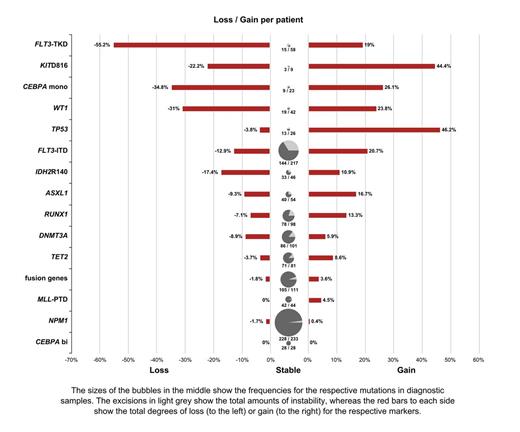
In 515/556 (92.6%) pts at least one mutation (mut) was detected at diagnosis (mean: 2.3; range:1-5). In detail, the most frequent markers were mut in: NPM1: n=233, FLT3-ITD: n=217, fusion genes: n=114, DNMT3A: n=101, RUNX1: n=98, TET2: n=81, FLT3-TKD: n=58, ASXL1: n=54, IDH2R140: n=46, MLL-PTD: n=44, WT1: n=42, TP53: n=26, biallelic CEBPA(bi): n=28, monoallelic CEBPA(mono): n=23. At relapse a similar incidence of 88.6% (558/630) mutated pts (mean: 2.0: range 1-6) was detected. However, the mutational pattern changed at relapse in 381/629 (60.6%) of the pts. On the marker level a change was seen for 309 (24.5%) of the mut, with gain of 142/1,263 new mut (11.3%) and a loss of 167 (13.2%) mut that were detectable at diagnosis.
Different degrees of stability were observed. Compared to all other markers a stable pattern was found for CEBPAbi: p=0.025; NPM1: p<0.001; MLL-PTD: p=0.006; and fusion genes: p<0.001. This is supporting the respective definition of entity defining mut in the WHO classification. Instead, a low stability (for gain and/or loss) compared to all others was seen for CEBPAmono: p=0.002, FLT3-ITD: p<0.001, FLT3-TKD: <0.001, NRAS: p<0.001, TP53: p<0.001 (only for gain), WT1: p<0.001. This is in accordance with the concept of typical “secondary” mutations, leading to acceleration of the disease. An intermediate stability was detected for TET2, DNMT3A, RUNX1, ASXL1,IDH2R140 (see figure).
Of note, of 200 FLT3-ITD positive pts that retained an FLT3-ITD at relapse, only 6 (3%) were stable with respect to mutational load whereas 134 (67%) showed an increase of the FLT3-ITD/wildtype load, and 60 (30%) a decrease showing a further quality of genetic instability.
Between the various disease entity defining groups there were no relevant differences in TTR. In contrast, an impact on shorter TTR was seen for mutations in DNMT3A (median TTR: 8.7 vs 12.1 months (m), p=0.058), FLT3-ITD (7.6 vs 12.2 m, p<0.001), RUNX1mut (10.1 vs 12.8 m, p=0.038) and TET2mut (9.9 vs 10.9 m, p=0.049). In the normal karyotype group an effect was only seen for FLT3-ITD (7.3 vs 13.6, p<0.001) and TET2mut (9.9 vs 12.3 m, p=0.025).
1) Genetic alterations in AML can be subdivided into stable, intermediate and unstable markers. Entity defining markers according to WHO (fusion genes, NPM1mut, CEBPAbi) are stable between diagnosis and relapse. 2) A significant negative impact on TTR was shown only for secondary mutations (FLT3-ITD, TET2mut, DNMT3Amut and RUNX1 mut).
Schnittger: MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Nadarajah:MLL Munich Leukemia Laboratory: Employment. Alpermann:MLL Munich Leukemia Laboratory: Employment. Eder:MLL Munich Leukemia Laboratory: Employment. Kohlmann:MLL Munich Leukemia Laboratory: Employment. Kuznia:MLL Munich Leukemia Laboratory: Employment. Weissmann:MLL Munich Leukemia Laboratory: Employment. Fasan:MLL Munich Leukemia Laboratory: Employment. Weber:MLL Munich Leukemia Laboratory: Employment. Albuquerque:MLL Munich Leukemia Laboratory: Employment. Jeromin:MLL Munich Leukemia Laboratory: Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Employment. Dicker:MLL Munich Leukemia Laboratory: Employment. Kern:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal