Abstract
Various forms of radiation (radioactive, UV) cause cellular damage, which may lead to premature cell death or accumulation of somatic mutations which may lead to initiation and progression of malignancy. The damage is mediated in part by free radicals, particularly reactive oxygen species (ROS) (Reizenstein, Med Oncol Tumor Pharmacother. 1991;8:229). Fermented papaya preparation (FPP) is a product of yeast fermentation of Carica papaya Linn. FPP has been shown to have anti-oxidative potential by scavenging ROS, as well as by chelating excess iron (Prus & Fibach J Biol Regul Homeost Agents. 2012;26:203). Free iron species, such as the labile iron pool (LIP), participate in chemical reactions that generate ROS (Haber-Weiss and Fenton reactions). We studied the potential effect of FPP in preventing radiation-induced damage to various intracellular components in cultured human fibroblasts and myeloid leukemia (HL60) cells and in mice.
Cultured cells, human foreskin fibroblasts and myeloid leukemia (HL-60), were irradiated at various doses (0-18 Gy (Gammacell® 220, MDS Nordion, Excel, Canada). FPP (Osato Research Institute, Gifu, Japan) (10-100 µg/ml, was added either before or after irradiation. The cells were assayed after 1-3 days: Their survival was estimated by CellTiter 96® Aqueous Non-Radioactive Cell Proliferation assay. Apoptosis was determined by staining for phosphatidylserine exposure (by fluorescent Annexin V) and for intracellular uptake of propidium iodide. ROS was measured by staining with dichlorofluoresceine diacetate, and the LIP - with calcein-AM. The effects on the DNA were estimated by measuring 8-oxyguanine (a marker of DNA oxidation), using a fluorescent specific probe, and DNA instability was measured by the “comet assay”, a single-cell gel electrophoresis procedure. Apoptosis, ROS, LIP and 8-oxyguanine were quantified by flow cytometry. Experiments were also carried out on irradiated mice treated with FPP (by adding it to the drinking water) either before or after irradiation. The above mentioned parameters were assayed in their bone marrow cells in addition to determining their survival.
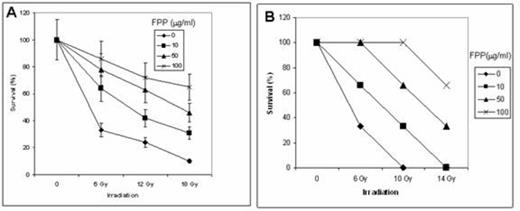
The results indicated that FPP has significant (P<0.05) ameliorating effects on radiation-induced increase of LIP and generation of ROS, as well as on the generation of 8-oxyguanine and DNA instability. In addition, apoptosis was decreased and consequently the survival of the cells was increased (Fig. 1A). Moreover, about 60% of 14 Gy-irradiated mice that received 100 mg/ml of FPP in their drinking water were still alive after 30 days (Fig. 1B).
Effect of FPP on irradiation-induced lethality of cultured normal human foreskin fibroblasts (A) and mice (B)
Effect of FPP on irradiation-induced lethality of cultured normal human foreskin fibroblasts (A) and mice (B)
FPP was able to protect the short-term effects of radiation on cultured cells by increasing their viability. A similar effect was found in mice. The improvement in DNA instability induced by FPP may influence the long-term effects of radiation, such as the development of secondary leukemia, in patients who receive radiotherapy for their primary tumors.
Fibach:Osato Research Institute, Gifu, Japan: Consultancy, Research Funding. Rachmilewitz:Osato Research Institute, Gifu, Japan: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal