Abstract
Resistance to ibrutinib (PCI-32765) is uncommon and not well described. Here we describe a 78 year old Caucasian man with a long standing history of CLL originally diagnosed in 2004. He was treated at the James Cancer Hospital and Fellow Research Institute at the Ohio State University since that time and received multiple regimens including fludarabine and Rituxan based regimens under the care of John Byrd, MD. He progressed and more recently was on the clinical trial of ibrutinib for relapsed CLL, which he tolerated well and to which he had an excellent response for nearly 2 years. The patient developed resistance to ibrutinib and was taken off study. According to Dr. Byrd, DNA sequencing revealed a V600 mutation (personal communication). The patient was subsequently enrolled on a clinical trial of carfilzomib, to which he did not respond and developed increasing leukocytosis with increasing numbers of prolymphocytes and transformation to B-cell PLL. He was treated with single agent ofatumumab with transient responses and rapid regrowth. He was then referred to the clinic at the Penn State Hershey State College satellite facility for care closer to his home.
Ibrutinib is a novel oral inhibitor of the bruton tyrosine kinase (BTK) which is a member of the Tec family of kinases and is downstream of the B-cell receptor. A Phase 1b-2 study of ibrutinib in patients with relapsed or refractory CLL or small lymphocytic lymphoma was recently conducted. Treatment with Ibrutinib was associated with durable remissions in this population including those with high-risk genetic mutations (Byrd et al NEJM 2013;369:32-42).
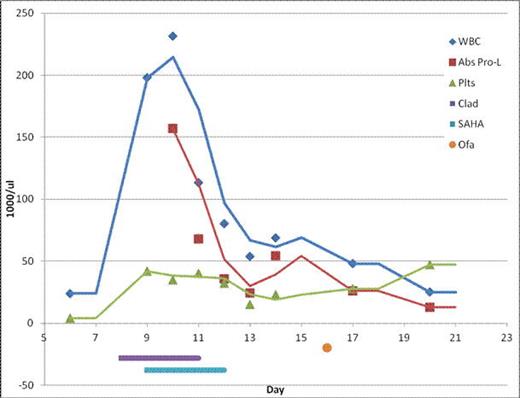
We have previously shown that immunotherapy combined with epigenetic pharmacotherapy is a feasible strategy in non-Hodgkin's lymphoma (NHL) and CLL in a phase 2 study (NCT 00764517) initiated by Epner and colleagues (Spurgeon et al 2012 ASH abstract 3675, Hasanli et al 2013 AACR LB-140) in which a 100% response rate was observed in untreated mantle cell lymphoma using SAHA (Vorinostat), cladribine and Rituxin (SCR). Based on these results, we proposed a treatment strategy which would employ the same epigenetic pharmacotherapy of SAHA and cladribine in combination with the immunotherapy of ofatumumab. Dr. Byrd agreed with our treatment strategy.
After 3 cycles of our epigenetic immunotherapy, he achieved a complete hematologic remission which is sustained for nearly 12 months. He receives ofatumumab maintenance therapy (2000mg IV every 60 days).
Here, we have used ofatumumab in combination with epigenetic pharmacotherapy in a ofatumumab refractory patient with CLL who acquired a 17p deletion to overcome ibrutinib resistance.
The mechanism of resistance to ibrutinib in this case and others has not been determined, but sequencing studies carried on in the lab of Dr. Byrd (personal communication) reveal the BRAF V600E mutation.
We describe here a patient with CLL who progressed through multiple therapies including ibritunib who achieved a complete hematologic response with the combination of SAHA, cladrbine, and ofatumumab. In this case, epigenetic modulation with pharmacologic intervention consisting of a hypomethylating agent in combination with a histone deacetylase may have enhanced the activity of ofatumumab and overcame ibrutinib resistance. Furthermore, our data suggests that the combination of epigenetic agents with monoclonal antibodies should be studied and that epigenetic agents are potentially capable of activating multiple silenced genes in different pathways. The epigenetic approach and use of hypomethylation agents in CLL deserve further investigations. This particular case demonstrates that (by current standards) a most promising drug (Ibrutinib) has some failures and the epigenetic approach may provide a strategy to salvage these patients.
Sharma:Celgene: Consultancy, Employment. Off Label Use: Cladribine: approved for Hairy Cell Lymphoma (HCL), used here to treat B-CLL Vorinostat: approved for Cutaneous T Cell Lymphoma (CTCL), used here to treat B-CLL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal