Direct oral anticoagulants (DOACs) have been developed to address some of the limitations of Vitamin-K antagonists (VKAs) and are claimed to be easier to use. However, deviations from the recommended use have been reported, sometimes leading to serious adverse events (Sorensen R et al. BMJ Open 2013, Hussain S et al. Eur J Hosp Pharm 2013, Lim SL et al. Med J Aust 2013, Legrand M et al. Arch Intern Med 2011, Bene J et al. Ann Pharmacother 2012). Our objective was to evaluate the appropriateness of prescribing DOACs in real-life clinical practice.
We conducted a prospective cohort study including patients admitted to a 450-bed teaching hospital (Belgium) from April to July 2013, who were taking rivaroxaban (Xarelto®) or dabigatran etexilate (Pradaxa®). A clinical pharmacist interviewed each patient to collect clinical and pharmaceutical data. Additional relevant information was retrieved from the electronic medical record. Appropriateness of prescribing was evaluated using the 10 criteria of the Medication Appropriateness Index (Samsa GP et al. J Clin Epidemiol 1994, Hanlon JT et al. J Clin epidemiol 1992). Explicit instructions were added, based on EU summary of the product characteristics and (inter)national guidelines. Due to limited cost-effectiveness data in Belgium, the tenth criterion of the MAI was not used.
The primary outcome measure was the prevalence of inappropriate prescribing, namely the proportion of patients with ≥1 inappropriate criterion. Secondary outcome measures included (a) a description of the main categories of inappropriateness prescribing, (b) comparison of results for patients on Xarelto® as compared to those on Pradaxa®, (c) the prevalence of adverse drug events and their relationship to inappropriate prescribing and (d) a description of interventions made by the clinical pharmacist to optimize prescribing.
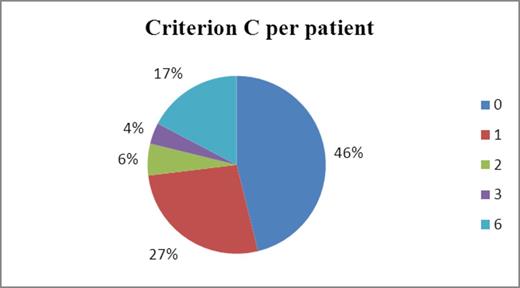
Fifty-two patients were evaluated (median age 74 years, range 51-89; 29 and 23 taking Xarelto® and Pradaxa®, respectively). Twenty-eight (53.8%) patients had at least one inappropriate rating: 1 inappropriate criterion in 26.9% and >1 in 26.9% of patients (Figure 1). The most frequent inappropriate criteria were: wrong dosage (32.7%, e.g. dose not adapted to renal function); inappropriate choice and modalities of administration (28.8%, e.g. prescription of a NOAC in a VKA-naïve patient with extreme body weight, once daily administration of Pradaxa®); and unpractical modalities of administration (25.0%, e.g. Pradaxa® in non-adherent patients) (Table 1). Results were overall similar when comparing Pradaxa® and Xarelto®, indicating that the trend of inappropriate prescribing might not be related to one specific DOAC. Twenty-four patients (47.0%) had experienced one adverse event (e.g. 8 patients had a stroke or a transient ischemic accident, 6 had hematomas or non-characteristic bleedings, 3 had epistaxis incidents and 2 patients reported dyspepsia); and 7.8% more than one adverse event. The clinical pharmacist made 29 interventions during the study period; e.g. 11 request for specific coagulation assay, 8 switches to another oral anticoagulant, 3 dose adjustments and 3 therapeutic education. Thirteen patients had one clinical pharmacist intervention and 8 patients benefited from 2 interventions.
Percentage of inappropriate ratings (C) per patient, using the Medication Appropriateness Index
Percentage of inappropriate ratings (C) per patient, using the Medication Appropriateness Index
Detailed ratings of appropriateness using the Medication Appropriateness Index (N)
| Criterion | A | B | C | Z | % C |
| <![if]>1. <>Indication | 38 | 5 | 9 | 0 | 17.3 |
| <![if]>2. <>Choice | 13 | 22 | 15 | 2 | 28.8 |
| <![if]>3. <>Dosage | 28 | 5 | 17 | 2 | 32.7 |
| <![if]>4. <>Administration | 24 | 11 | 15 | 2 | 28.8 |
| <![if]>5. <>Practicability | 37 | 0 | 13 | 2 | 25.0 |
| <![if]>6. <>Drug-drug interaction | 4 | 47 | 1 | 0 | 1.9 |
| <![if]>7. <>Drug-disease interaction | 43 | 8 | 1 | 0 | 1.9 |
| <![if]>8. <>Duplication | 51 | 1 | 0 | 0 | 0.0 |
| <![if]>9. <>Duration | 43 | 0 | 9 | 0 | 17.3 |
| Criterion | A | B | C | Z | % C |
| <![if]>1. <>Indication | 38 | 5 | 9 | 0 | 17.3 |
| <![if]>2. <>Choice | 13 | 22 | 15 | 2 | 28.8 |
| <![if]>3. <>Dosage | 28 | 5 | 17 | 2 | 32.7 |
| <![if]>4. <>Administration | 24 | 11 | 15 | 2 | 28.8 |
| <![if]>5. <>Practicability | 37 | 0 | 13 | 2 | 25.0 |
| <![if]>6. <>Drug-drug interaction | 4 | 47 | 1 | 0 | 1.9 |
| <![if]>7. <>Drug-disease interaction | 43 | 8 | 1 | 0 | 1.9 |
| <![if]>8. <>Duplication | 51 | 1 | 0 | 0 | 0.0 |
| <![if]>9. <>Duration | 43 | 0 | 9 | 0 | 17.3 |
(A) Appropriate (B) Inappropriate but with little clinical importance (C) Inappropriate (Z) Not enough information to evaluate appropriateness
The quality of prescribing DOACs was suboptimal. Off-label use was frequent and suggests that reinforcing education of health care professionals is needed. This should be a priority for the future.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal