Tacrolimus is a widely used calcineurin inhibitor in allogeneic HCST for prophylaxis and treatment of GvHD. This molecule is now available in both standard-release (Prograf, twice-daily tacrolimus, Astellas Pharma) and extended-release (Advagraf, once-daily tacrolimus, Astellas Pharma) formulations. Compared with the standard-release formulation, the extended-release (ER) tacrolimus has been shown in previous pharmacological studies to provide bioequivalent drug exposure, efficacy and safety. Moreover, this formulation of tacrolimus was followed by a clinically significant improvement in kidney graft function for kidney recipients. Based on these observations, we have conducted a prospective study in wich ciclosporine A (CsA) (Neoral) is switched for tacrolimus ER (Advagraf) in case of renal impairment after alloHSCT.
We enrolled 16 consecutive patients with renal impairment (serum creatinine >110 µMol/L) from May 2012 to May 2013 during the follow-up at outpatient clinic, the conversion dose was established on an mg:mg basis 1:100 from CsA total oral daily dose to a single dose of oral tacrolimus ER formulation. The dose was readjusted to obtain a tacrolimus blood trough level between 5 and 15 µg/L. Tacrolimus ER was given at noon in a single dose one hour before lunch. We evaluated the tacrolimus blood trough level changes after switch, serum creatinine, glycemia, potassium, acute and chronic GvHD. A satisfaction survey for tacrolimus ER treatment was performed 3 months after the switch, the questionnaire included administration compliance questions.
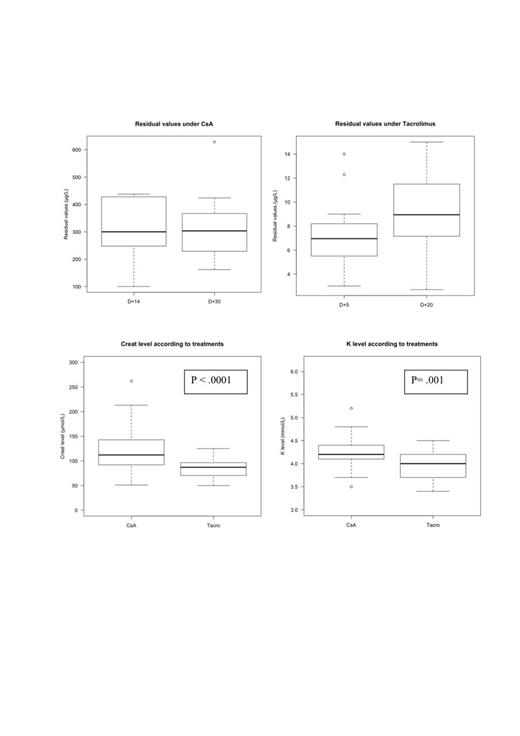
All patients in this cohort had hematological malignancies, the median age was 52 years (range, 28-66). Eight patients (50%) had a matched related donor, 7 patients (43.75%) had a HLA-10/10 matched unrelated donor and 1 patient underwent alloHSCT with two cord blood units. Seven patients (43.75%) received a myeloablative regimen with 12 Gy TBI and 9 patients (56.25%) a reduced intensity regimen. The stem cell source was bone marrow for 7 patients (43.75%), PBSC for 8 patients (50%) and cord blood for one patient. The status at transplantation was CR1 for 6 patients (37.5%), CR2 or more for 6 patients (37.5%) and partial response for 4 patients (25%). The median follow-up of the cohort was 7 months (range,2-14). Non-parametric tests such as exact Wilcoxon Mann-Whitney test or Kruskal Wallis were performed for the analysis of the physiological parameters. The median of serum creatinine was 112 µmol/L (range, 51-262) with CsA and 87 µmol/L (range, 50-125) with tacrolimus ER (p<.0001) (Figure). The median of potassium level was 4.2 (range, 3.5-5.2) with CsA and 4.0 (3.4-4.5) with tacrolimus ER (p =.001) (Figure). The median time of switch was 45 days (10-162) and the median of serum creatinine in which the switch was realized was 138 µmol/L (range,110-262). Nor patient had diabetes mellitus neither elevation of potassium > 4.5 mmol/L after the switch for tacrolimus ER. The median blood trough level was 300 µg/L (range,100-438) for CsA 14 days after starting and 7.2 (range,3-15) for tacrolimus ER 20 days after starting (Figure). The cumulative incidence of aGvHD grade >1 at 3 months was 25% (95%CI,14-36). After the switch for tacrolimus ER, no patient developped aGvHD. Eight patients (50%) developed aGvHD grade >1 during the prophylaxis with CsA. For 6 patients (37.5%), aGvHD was resolutive after the switch for tacrolimus ER with an association of tacrolimus ER and prednisone or tacrolimus ER alone. For 2 patients (12.5%), aGvHD was resolutive with CsA and prednisone before the switch for tacrolimus ER. Two patients in this study developed severe chronic GvHD after the discontinuation of prophylaxis (5 months and 10 months). One patient received tacrolimus ER with a complete resolution of cGvHD and the second patient is now on therapy with everolimus. All patients in this cohort are alive and all patients except two are still in complete response. Moreover, the satisfaction survey demonstrated that the patients were satisfied with the switch and the one daily formulation of tacrolimus.
The conversion from oral CsA to oral tacrolimus ER formulation was followed by a clinically significant improvement in kidney function with stable tacrolimus blood trough levels. The patients were satisfied with this formulation of tacrolimus. We have now extended this study to several centers in the aim to confirm these observations.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal