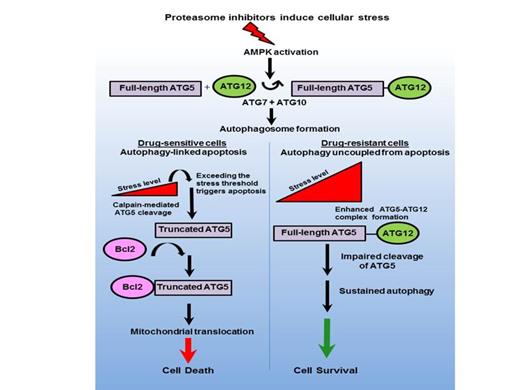
Cancer cells face enormous metabolic challenges to meet energetic and biosynthetic demands and, therefore, a hallmark of cancer cells is reprogrammed metabolic circuitry that results from oncogenic events selected during tumorigenesis to promote growth, survival and drug resistance. The increased metabolic demands in tumor cells are offset by autophagy - a basic catabolic mechanism that involves cell degradation of unnecessary or dysfunctional cellular components and potentially contributes to the growth of cancer cells. The proteasome is the catalytic core of the ubiquitin (Ub)-dependent proteolytic pathway that degrades short-lived and denatured proteins to maintain cell viability. Success of the proteasome inhibitor bortezomib has emerged as standard-of-care therapy for the invariably fatal malignancy MM to catapult the Ub+proteasome system (UPS) into a position of prominence in cancer biology and drug discovery, significant obstacles remain since many patients do not respond to bortezomib and those that do inevitably develop drug resistance through mechanisms that remain elusive. We discovered that in multiple myeloma (MM) cells, bortezomib triggers activation of AMPK - the master regulator of cellular energy metabolism. At physiologically-relevant concentrations, proteasome inhibitors further triggered AMPK-dependent autophagosome formation in myeloma cells that was directly and sequentially linked to apoptosis in drug-sensitive cells. However, in bortezomib-resistant cells, autophagosome formation and apoptosis were uncoupled to enhance drug resistance and the survival of tumor cells. Genetic knockout of the AMPK catalytic subunits in mouse embryonic fibroblasts reduced the effect of bortezomib on both autophagy and apoptosis. Similar effects were seen with genetic ablation of ULK1- a downstream substrate of AMPK - also required for bortezomib-induced autophagosome formation. Enforced expression of the autophagy-related gene Atg5 enhanced bortezomib-induced cell death while bortezomib treatment promoted ATG5 cleavage to yield a truncated, pro-apoptotic form. ATG5 participates in the initial steps of autophagy and was also cleaved by calpain to generate the truncated ATG5 form that translocates to the mitochondria, binds Bcl2 and induces mitochondrial outer membrane permeabilization (MOMP). We propose that ATG5 promotes the displacement of anti-apoptotic Bcl2 proteins from the pro-apoptotic Bcl2 family members, e.g., NOXA and PUMA, to facilitate MOMP - considered the point of commitment to cell death through the mitochondrial apoptotic pathway. Importantly, bortezomib in combination with the AMPK activators AICAR or metformin enhanced ATG5 cleavage and overcame resistance to proteasome inhibitors. The molecular events that regulate the complex interplay between autophagy and apoptosis as determinants of cell fate under physiologic and pathologic conditions have remained poorly understood. Since there is an urgent need to unravel the mechanism(s) of drug resistance and to develop more effective therapies, we propose pharmacologic repositioning of AMPK activators as a promising strategy to enhance the therapeutic efficacy of proteasome inhibitors to overcome drug resistance in the treatment of hematologic malignancies.
Disclosures:
No relevant conflicts of interest to declare.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal