Natural killer (NK) cells mediate anti-lymphoma activity by spontaneous cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) when triggered by rituximab, an anti-CD20 monoclonal antibody (mAb) used to treat patients with B cell lymphomas. The balance of inhibitory and activating signals determines the magnitude of NK cell's efficacy by spontaneous cytoxicity. Using a killer cell immunoglobulin-like receptor (KIR) transgenic murine model, we show that blockade of the interface of inhibitory KIRs with MHC class I antigens on lymphoma by anti-KIR antibodies prevents a tolerogenic interaction and augments NK cell spontaneous cytotoxicity. In combination with anti-CD20 mAbs, anti-KIR treatment induces enhanced NK cell-mediated, rituximab-dependent cytotoxicity against lymphoma in vitro and in vivo in syngeneic and KIR transgenic murine lymphoma models.
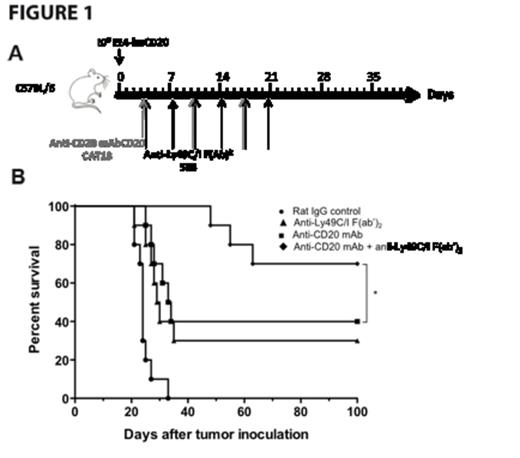
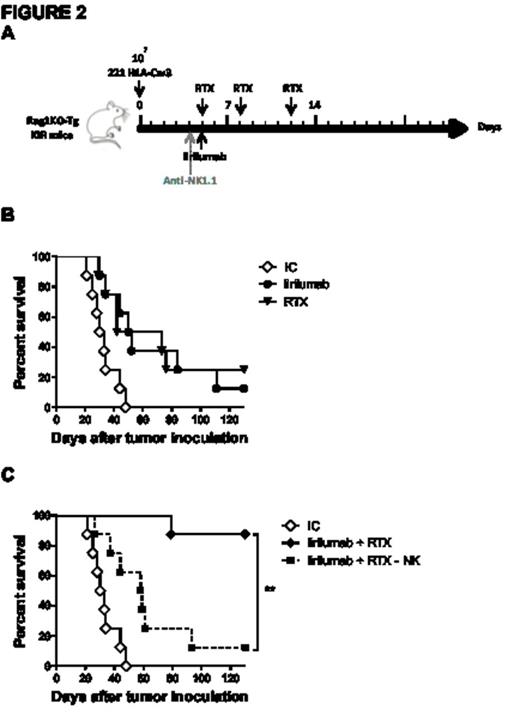
Specifically targeting murine NK cells in vitro, anti-Ly49C/I F(ab')2 increased anti-CD20 mAb-mediated NK cell degranulation as measured by CD107a mobilization and interferon-γ release, as well as increased cytotoxicity as assessed by chromium release. In the syngeneic EL4-huCD20 lymphoma model, anti-Ly49C/I F(ab')2 enhanced the anti-lymphoma activity of anti-CD20 mAb in vivo (Fig 1A-1B) and was NK cell-dependent with efficacy abrogated by NK cell depletion with anti-Asialo-GM1. To validate these observations and the potential efficacy of a fully human anti-KIR mAb (IPH2101, lirilumab), we demonstrated, in vitro, dose-dependent KIR2DL3 saturation and tumor lysis following blockade of KIR2DL3/HLA-C with lirilumab. In the transgenic KIR murine model, lirilumab therapy improved survival in an NK cell-dependent manner in both a prophylactic and therapeutic HLA+ (221 HLA-Cw3) lymphoma model. In combination, lirilumab therapy synergistically enhanced rituximab's anti-lymphoma efficacy in vivo in an NK cell-dependent manner (Fig 2A-C).
These results support a therapeutic strategy of combination, rituximab and KIR blockade through lirilumab, illustrating the potential efficacy of combining a tumor targeting therapy with an NK cell agonist thus stimulating the post-rituximab anti-lymphoma immune response.
Thielens:Innate Pharma: Employment, Equity Ownership. Sola:Innate Pharma: Employment, Equity Ownership. Chanuc:Innate Pharma: Employment, Equity Ownership. Fuseri:Innate Pharma: Employment. Bonnafous:Innate Pharma: Employment, Equity Ownership. Vivier:Innate Pharma: Membership on an entity’s Board of Directors or advisory committees. Romagne:Innate Pharma: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees. Andre:Innate-Pharma: Employment, Equity Ownership. Blery:Innate Pharma: Employment, Equity Ownership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal