Abstract
Radioimmunoconjugates are effective treatment in relapsed/refractory follicular lymphoma (FL) when used as single agents, and can result in effective disease control (Kaminski et al, NEJM 1993; Witzig, JCO 2002). When used as consolidative treatment following a course of initial chemotherapy for patients with newly diagnosed FL, durable remissions have been noted (Gordon LI et al, Blood 2004). As a pure high energy beta emitting isotope, Zevalin has several advantages and has been well studied in relapsed refractory indolent NHL (Witzig TE et al, JCO 2002). Therefore, our hypothesis is that radioimmunoconjugates significantly change outcome for patients with FL when given in a situation of minimal residual disease, and number of long term remissions would increase with initial cytoreduction.
For cytoreduction, we chose to use an outpatient formulation of ESHAP (etoposide, methylprednisolone, cytarabine, cisplatin): ESHAP has excellent efficacy as a salvage regimen in treating relapsed low-grade lymphomas (Rodriguez-Monge EJ et al, Hem Oncol Clin N Am, 1997). Further, it is not utilized by community oncology practices fostering chemo sensitivity in a relapsed setting.
Histologically confirmed CD 20 + relapsed FL, ≥1< 4 prior therapies, age 18, ECOG performance status 0–2, measurable disease, signed informed consent, creatinine, bilirubin< 2.0 x ULN , platelet counts ≥150,000 able to receive 0.4 mCi/kg of Zevalin; patients with platelet counts 100,000-150,000 received 0.3mCi/kg dose of Zevalin. Patients were treated with 2 cycles of ESHAP every 28 days. At subsequent restaging, if bone marrow aspirate, biopsy showed<25% involved and expected biodistribution, Zevalin was administered. If Bone marrow involvement was >25 % was noted, patients were taken off study secondary to treatment failure.
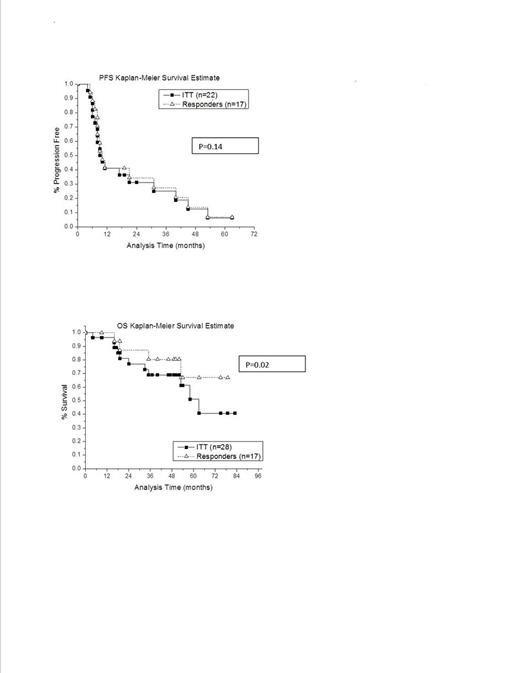
Twenty-eight patients with FL were enrolled with total 8 year follow-up. 6 patients did not complete the study: one patient was ineligible secondary to re-review of path showing DLBCL. Three patients were ineligible for study completion secondary to bone marrow showing residual involvement. Two patients withdrew secondary to toxicities: one from a perforated duodenal ulcer (SAE), and one from side effects of ESHAP chemotherapy including nausea, progressive functional decline. Other Grade 3, 4 adverse events included myelosuppression. Twenty-two patients were evaluable for response. Of the 22 evaluable patients, the overall response rate was 72% (17/22) with another 13 % achieving stable disease. After follow-up of 8 years, the median progression free survival (PFS) was 10 months for both the intent to treat analysis and responders (p=0.14). The median overall survival (OS) in the intent to treat analysis was 63 months, and the median OS in the 8 year follow-up of responders has not been reached (p=0.02). When analyzed by median number of prior therapies, the median PFS for patients with more than one prior chemotherapy regimen was 9, whereas the median PFS for patients with one prior regimen was 22 months. Similarly, for patients with more than one prior therapy, the median OS was 54 months, whereas the median OS for patients with one prior regimen has not been reached at 8 year follow-up.
In prior long term follow-up data(7 years) of a phase I/II study of Zevalin (Gordon et al, Blood 2004) in NHL including FL, the median time to progression in responders was 12.6 months, and durable responses were noted in 5/51 patients with FL. In a phase II study of relapsed FL treated with Zevalin (Witzig et al, JCO 2002) the median time to progression was 6.8 months, and in a subsequent Phase III study comparing Zevalin to rituximab, the median time to progression was 11.2 months.
In comparison to above studies, our study has shown that outpatient ESHAP is an effective cytoreductive regimen. Zevalin is active when administered in a setting of minimal residual disease early in the disease course as evidence by the excellent overall survival of the responders.
Off Label Use: Use of the investigational agent MLN8237 in combination in patients with aggressive B-cell NHL. Persky:Millennium: The Takeda Oncology Company: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal