Abstract
Great efforts have been made in the last years to improve the therapeutical options of patients with mantle cell lymphoma (MCL), a distinct lymphoma subtype characterized by dismal long term prognosis. Although radioimmunotherapy (RIT) has been shown to be effective in follicular lymphoma, only one single-center experience of 31 evaluable patients has been published so far in relapsed MCL [Wang et al., JCO 2009]. The European MCL Network conducted a phase II, prospective, multicenter trial evaluating a single dose of yttrium-90-ibritumomab tiuxetan (90Y-IT) as reinduction or consolidation in patients with relapsed or refractory MCL.
Relapsed or refractory MCL patients after or not eligible for autologous stem cell transplantation (ASCT) with<25% bone marrow involvement received a single infusion of rituximab (250 mg/m2, day 1) followed by a sequential infusion of rituximab and 90Y-IT 0.4 mCi/kg on day 8 (0.3 mCi/kg if thrombocytes<150000/μl or prior ASCT: group A). In patients with high tumor load a short prior immuno-chemotherapy (e.g. 3 cycles of rituximab-bendamustin) was allowed (group B). The primary study objective was response rate (complete/partial responses, CR/PR), secondary objectives were progression-free survival (PFS), overall survival (OS), as well as safety of 90Y-IT.
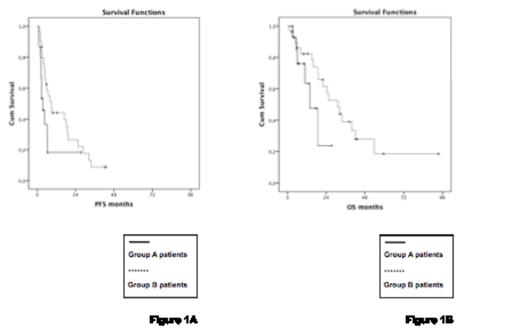
Between June 2004 and September 2008, 48 eligible patients were enrolled (16 group A, 32 group B) and 45 are evaluable for response. Median age was 68 years, 75% were males. Seventy-three % presented with high or intermediate risk MCL international prognostic index (MIPI), 42% with elevated lactate dehydrogenase, and 29% had bulky disease. Median number of previous therapies was 2 (range 1-5) in group A and 4 (range 1-6) in group B; 98% of patients received prior rituximab, 29% prior radiotherapy, 13% prior ASCT, 0% prior new agents and 15% had chemorefractory disease. The major toxicities consisted of myelosuppression, with thrombocytopenia in 21 patients (53%), neutropenia in 13 (33%) and anemia in 9 (23%; all grade 3/4, respectively), and one lethal bleeding. Non-hematologic grade 3/4 toxicities were gastrointestinal (n=3), infectious (n=1), and neurological (n=1), with a single patient (2%) developing a secondary myelodysplasia. Overall response rate (ORR) was 40% (20% CR) in group A and 72% (38% CR) in group B, with 5 patients converting from PR to CR. After a median follow-up of 38 months (range: 24-53 months), median PFS was 3.7 months in group A and 8.9 months in group B, translating in a median OS of 13.8 months and 31.2 months, respectively (Figure 1). In the 25 90Y-IT responders, PFS and OS were 23 months and 33.7 months, respectively, and patients responding to immunochemotherapy (group B) also showed a more favorable time to progression (16.9 months). No difference in survival rates was noticed according to MIPI, bulky disease, number and type of previous therapies, chemorefractoriness, and median time from initial diagnosis.
PFS and OS of patients in group A and B after receiving 90Y-IT
PFS and OS of patients in group A and B after receiving 90Y-IT
To the best of our knowledge, this is the largest trial assessing 90Y-IT therapy in relapsed/refractory MCL patients. Response rates in heavily pre-treated patients were comparable to those of other targeted approaches, though 90Y-IT showed a more favorable toxicity profile. Our experience suggests, that RIT applied as consolidation therapy might be the preferred approach, seeming to improve the quality and duration of response. Finally, responses appear to be independent from established risk factors and previous therapies. Further evaluation of the role of 90Y-IT in first-line therapy of MCL patients is ongoing.
Off Label Use: Zevalin for treatment of relapsed mantle cell lymphoma. Scholz:Bayer: Speakers Bureau; Spectrum: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau. Keller:Sepropharm: Consultancy. Dreyling:Jannsen: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau, support of ITS, support of ITS Other; Celgene: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau, support of ITS Other; Pfizer: Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau, support of ITS , support of ITS Other; Roche: Speakers Bureau, support of ITS , support of ITS Other; Mundipharma: support of ITS Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal