Abstract
The prognosis of young DLBCL patients at high risk treated with standard R-CHOP is still rather poor. With the aim to improve outcome of these patients the Italian (Vitolo U et al, Blood, ASH annual Meeting 2012) and German (Schmitz N et al, Lancet Oncology 2012:13,1250) cooperative study groups conducted two randomized phase III studies aimed to compare conventional chemotherapy with intensified chemotherapy with stem cell support both in combination with Rituximab (R). The standard reference arms were R-CHOP14 in the Italian and R-CHOEP14 in the German study. No randomized studies comparing R-CHOP14 and R-CHOEP14 exist. Therefore, FIL and DSHNHL did a joint analysis of the two randomized trials, with the aim to compare the two conventional arms R-CHOP14 and R-CHOEP14.
The primary endpoint of this joint analysis was to compare the progression free survival (PFS) of DLBCL patients treated with R-CHOP14 or R-CHOEP14 within the two prospective trials. Inclusion criteria were superimposable for the two studies as follows: age 18-60 years; untreated CD20+ DLBCL or follicular lymphoma grade IIIb; age-adjusted IPI (aa-IPI) score 2 or 3. FIL patients were treated with R-CHOP14 for 8 courses; DSHNHL patients were treated with R-CHOEP-14 for 8 courses. Involved-field radiotherapy (IF-RT) for patients with extranodal sites or with bulky disease was based on the investigator choice in the FIL trial and was mandatory in the DSHNHL study. Central nervous system prophylaxis was done in patients at risk for CNS recurrence. Response criteria used were the same in both studies (Cheson 1999).
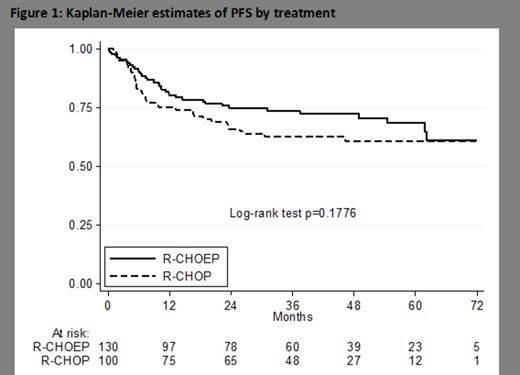
From June 2005 to September 2010 100 patients were enrolled in the FIL study and treated with R-CHOP14. From March 2003 to April 2009, 130 patients were enrolled in the DSHNHL study and treated with R-CHOEP14. All 230 patients were included in the present analysis. Clinical characteristics were: median age 49 (IQR 38; 55); stage III/IV 96%; LDH > normal 93%; ECOG PS >1 38%; aa-IPI score 2/3 74/26%; extranodal sites >1 41%, bulky disease 50% and bone marrow (BM) involvement 16%. All characteristics were well balanced between patients treated with R-CHOP14 or R-CHOEP14 with the exception of significantly more patients with elevated LDH and bulky disease in the R-CHOEP14 group (98% vs 87%; 59% vs 37%) and more patients with PS >1 in the R-CHOP14 group (45% vs 32%). IF-RT consolidation was less frequently used in the R-CHOP14 group (16% vs 74%). Median follow-up times were: 47 (R-CHOP14) and 42 (R-CHOEP14) months. Patients treated with R-CHOP14 had a 3-year PFS of 63% (95% CI:52-71%) compared to 74% of those treated with RCHOEP14 (95% CI:65-81%); p = .177 (Figure 1). In a Cox model for PFS including treatment arm, age, gender, aaIPI, extranodal sites, bulky disease and BM involvement the adjusted HR was 0.68 (95% CI: 0.42-1.2) in favor of RCHOEP14, but this difference was not significant (p = .128). The analysis of the role of consolidation IF-RT is ongoing.
This analysis comparing R-CHOP14 and R-CHOEP14 as standard arms in two prospective trials suggests that RCHOEP14+RT has high activity in young poor-risk DLBCL. To confirm these encouraging findings, a formal joint FIL/ DSHNHL randomized study comparing R-CHOEP+RT vs R-CHOP+RT +/- novel drugs is planned by the two cooperative groups.
Vitolo:Roche: Speakers Bureau; Celgene: Speakers Bureau; Takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal