Abstract
Waldenström's macroglobulinemia (WM) is a B-cell lymphoproliferative disorder characterized by a lymphoplasmacytic infiltration of the bone marrow (BM) and a serum monoclonal IgM. WM tumor cells show variable differentiation, ranging from mature B-cells to plasma cells. T-cell leukemia/lymphoma 1 (TCL1) is a small non-enzymatic protein normally expressed only during the early stages of lymphopoiesis and known to be a coactivator of AKT and of the NF-KappaB transcription pathway. TCL1 is up-regulated and plays a critical role in the pathogenesis of chronic lymphocytic leukemia (CLL) since transgenic mice expressing TCL1 in B cells developed an aggressive form of CLL (Bichi et al., PNAS 2002). Moreover high TCL1 expression level is linked to aggressive features and poor outcome in CLL. TCL1 was also shown to be expressed in various B-cell lymphomas but not in multiple myeloma (Weng et al., Blood 2012). TCL1 might also have a potential role in the pathogenesis of WM but TCL1 expression is not so far described in WM.
We proposed to assess the expression pattern of TCL1 and its potential prognostic value in WM.
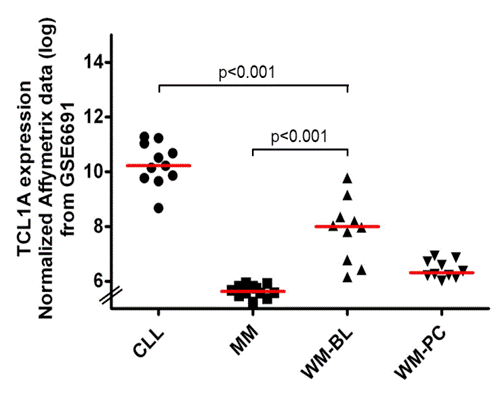
Firstly, we checked the TCL1A expression in WM by conducting an analysis using NCBI’s GEO2R tool of publically available transcriptomic data which compared gene expression profiling of CLL (n=11), MM (n=12) and WM patients (B-lymphocyte (BL) (n=10) and plasma cell (PC) (n=10)) (GSE6691 from NCBI’s GEO database, Gutiérrez et al. Leukemia 2007). Secondly, we performed histological and immunostaining analyses for TCL1 on deparaffinized whole tissue contiguous sections of 57 WM patients’ BM trephine biopsies (median age: 62 years, sex ratio M/F: 36/21, symptomatic: n=36, previously treated: n=10). Diagnostic and treatment criteria for WM fulfilled recommendations of the 2nd international WM workshop. TCL1 immunostains were graded independently by three investigators on a 4-tier scoring which had previously been used to study TCL1 in CLL (Herling et al., Leukemia 2006). TCL1 immunostains intensity was scored as follows: 1- low intensity, 2- middle intensity, 3- strong intensity; tumor infiltration patterns were defined as interstitial, nodular and interstitial, nodular or diffuse and tumor infiltration percentage was assessed according to CD20 immunostains.
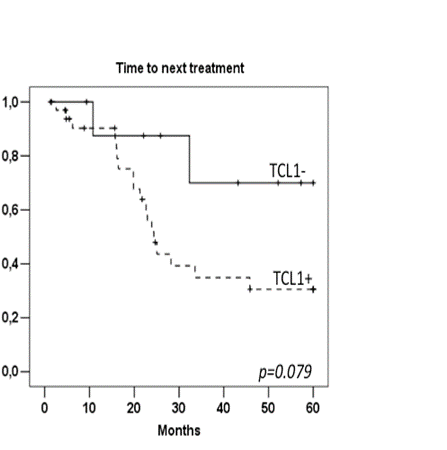
The analysis of transcriptomic data revealed that TCL1A expression level in WM is significantly higher than those found in MM and lower than those found in CLL (Figure 1). Immunohistochemistry analysis of BM showed that TCL1 protein was expressed in 45 patients (79%), with an intratumoral heterogeneous repartition and its localization appeared mostly cytoplasmic. Pattern of expression was: negative (n=12), less than 5% of tumor cells positive (n=6), positive in up to 50% of tumor cells (n=15), and positive in over 50% of tumor cells (n=24). TCL1 pattern was correlated neither with tumor infiltration pattern nor grading. TCL1 seemed not to be associated with any prognostic factors and TCL1+ and TCL1- patients were comparable in terms of clinical and lab features as for ISS. Median follow-up was 50 months. Among the 21 asymptomatic WM patients, median time to first treatment was 33 months for TCL1+ group, while none of TCL1- patients needed so far any first line therapy (p=0.24). Among the patients requiring treatment, we did not observe any impact of TCL1 expression level on ORR (63% for TCL1+ and 67% for TCL1- patients, p=0.85). TCL1+ patients presented a trend for a poorer 3 year-PFS than TCL1- patients (32% versus 62% respectively, p=0.15). Median time to next treatment (TTNT) was only 24 months for TCL1+ patients and not reached for TCL1-patients (p=0,079) (Figure 2). Median OS was 94 months in the whole population without clear influence of the TCL1.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal