Abstract
Cold agglutinin disease (CAD) is an autoimmune hemolytic anemia in which autoantibodies bind to red blood cells (RBC) at temperatures below 37°C, resulting in activation of the classical complement pathway (CCP). CCP activation leads to hemolysis either intravascularly, by formation of the membrane attack complex, or extravascularly, when C3/C4 fragment deposition onto the RBC surface results in sequestration by the reticuloendothelial system. Here we describe the in vitro and in vivo activity of TNT003 and TNT009, inhibitors of a serine protease specific to the CCP, in pre-clinical models of CAD.
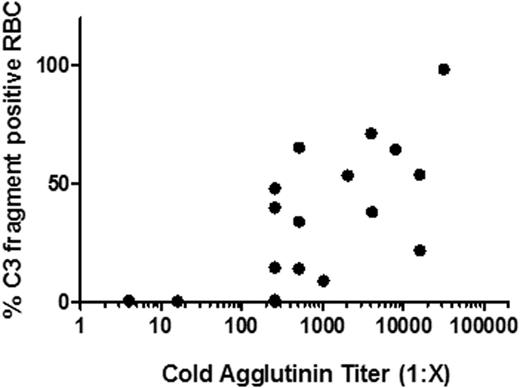
TNT003 is a mouse monoclonal IgG2a antibody with sub-nanomolar affinity. TNT009 is the humanized form (IgG4) of TNT003 and retains affinity and specificity to its target. In vitro assays using IgM-sensitized sheep RBC and human or non-human primate (NHP) serum showed that TNT003 and TNT009 potently inhibited antibody-mediated hemolysis in a concentration dependent manner. Additionally, TNT003 and TNT009 inhibited CCP-mediated production of the anaphylatoxins C4a, C3a, and C5a. Flow cytometry analysis showed that both antibodies also prevented C3 fragment deposition on the RBC surface. Activity was proportional to the amount of serum used, and at 80% human or NHP serum, TNT003 completely inhibited hemolysis with an IC50 of ∼13 µg/mL. Using an ELISA-based assay, TNT003 inhibited C5b-9 deposition driven by the CCP but not by the alternative (CAP) or lectin (CLP) pathways. These data suggest that TNT003 and TNT009 are specific and potent inhibitors of the CCP.
Extravascular hemolysis of C3 fragment-coated RBC by liver macrophages is believed to be the primary mechanism of RBC destruction in CAD. We therefore tested the hypothesis that CAD patient plasma-induced C3 fragment deposition on RBC would promote phagocytosis by the monocytic cell line THP-1. We found that RBC sensitized in CAD plasma and exposed to NHS were engulfed in an FcgR-independent mechanism by THP-1 cells. RBC phagocytosis was significantly inhibited if NHS exposure occurred in the presence of TNT003 (100 µg/mL), but not a control IgG.
The selective CCP inhibitory activity of TNT003 was evaluated in vivo in cynomolgus monkeys. TNT003 administered as a single IV injection at 30 mg/kg resulted in a Cmax of ∼330 µg/mL and detectable serum TNT003 thru ≥72 hours. Using an ELISA-based assay, we observed specific inhibition (≥95%) of the CCP for ≥72 hours. In contrast, CAP activity was modestly and transiently inhibited for 4 - 8 hours. At Cmax, endogenous C4a levels were reduced by >90% and returned to baseline levels by ≥96 hours. Serum samples containing TNT003 showed complete (100%) inhibition of hemolysis and C3 fragment deposition in vitro. CCP activity was completely restored to baseline after TNT003 concentrations fell below a predictable, threshold level.
Collectively, these data indicate that TNT003 and TNT009 are potent and specific inhibitors of CCP activity and C3 fragment deposition in vitro and in vivo. These findings support the preclinical development of TNT009 for the treatment of CCP-mediated diseases including CAD.
Panicker:True North Therapeutics, Inc.: Employment, Equity Ownership. Shi:True North Therapeutics, Inc.: Employment, Equity Ownership. Rose:True North Therapeutics, Inc.: Employment, Equity Ownership. Hussain:True North Therapeutics, Inc.: Employment, Equity Ownership. Tom:True North Therapeutics, Inc.: Employment, Equity Ownership. Strober:True North Therapeutics, Inc.: Employment. Sloan:True North Therapeutics, Inc.: Consultancy. Parry:True North Therapeutics, Inc.: Employment, Equity Ownership. Stagliano:True North Therapeutics, Inc.: Employment, Equity Ownership, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal