Abstract
Conflicting results have emerged, especially with respect to the impact on overall survival (OS), from trials evaluating lenalidomide maintenance (LM) therapy after induction therapy alone or post-autologous stem cell transplant (ASCT) in multiple myeloma (MM). We performed a systematic review and meta-analysis of existing outcome data from LM trials to evaluate role of lenalidomide as maintenance strategy in MM.
A comprehensive search of electronic databases and abstracts through June 2013 was performed to identify randomized controlled trials (RCTs) that compared LM vs. placebo/no maintenance. Single arm studies were excluded. Pooled hazard ratio (HR) or odds ratio (OR) estimates with 95% confidence intervals (CIs) were calculated using the random-effects model for clinical endpoints of progression free survival (PFS), OS, response rate (RR) and adverse events (AEs), including second primary malignancies (SPMs). Analyses were performed using Comprehensive Meta-Analysis Software Version 2. We assessed between-study heterogeneity with the Cochran Q test and quantified its extent with the I2 statistic.
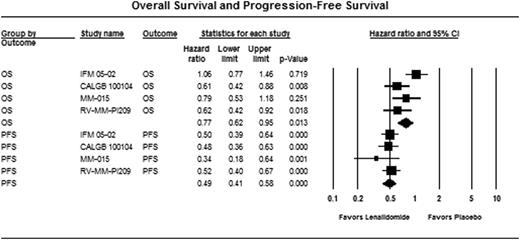
Overall, five RCTs, with data extractable from four phase III trials (3 publications and 1 abstract) were identified (n= 1935). All studies were RCTs with an adequate randomization. MRC MM XI study was excluded from analyses as survival data are not available. Two placebo controlled trials (IFM 05-02, CALGB 100104) addressed the role of LM post-ASCT, one placebo-controlled trial (MM-015) studied LM therapy in the non-transplant setting and the remaining trial (RV-MM-PI209) had a 2 X 2 design comprising of both ASCT and non-transplant randomized arms followed by a second randomization of LM versus no maintenance. There was no heterogeneity for estimate of PFS results (Cochran Q, p=0.68; I2=0%), but considerable heterogeneity for estimate of OS (Cochran Q, p=0.09; I2= 55%), among the studies. There was significant prolongation of both PFS (HR 0.49, 95% CI, 0.41–0.58, p<0.001) and OS (HR 0.77, 95% CI, 0.62–0.95, p=0.013) with LM vs. placebo/no maintenance (Figure 1). Best response during maintenance was reported only in 2 studies and odds of responding (very good partial response or better) were not significantly different with LM (OR 1.28, p=0.3). Grade 3-4 AEs data were available from 3 trials for calculation of pooled OR with LM compared with placebo. We observed a nearly two-fold increase in the risk of SPMs with LM (OR 1.99; 95% CI, 1.31–3.04; p=0.001). Patients on LM were more likely to have grade 3-4 AEs than placebo: neutropenia (OR 4.9, p<0.001), thrombocytopenia (OR 2.7, p<0.001), fatigue (OR 2.3, p=0.01) and venous thromboembolism (OR 3.2, p=0.02). Odds of discontinuing treatment were also significantly higher in patients on lenalidomide (OR 2.9, p<0.001).
Meta-analysis of RCTs demonstrates significant improvement in PFS and modest improvement in OS with LM. There is an increased risk of grade 3-4 adverse effects, including SPMs with LM. Substantial heterogeneity for estimate of OS among protocols is a limitation of this analysis. Lack of uniform access to lenalidomide upon disease progression in the placebo/no maintenance arms of the constituent studies should be taken into account while interpreting aggregate effect estimates for OS in this meta-analysis.
OS: Cochran Q p=0.09, I2=55%, substantial heterogeneity
PFS: Cochran Q p=0.68, I2=0%, minimal heterogeneity
Off Label Use: Lenalidomide for maintenance therapy in multiple myeloma. Kumar:Merck: Consultancy, Honoraria; Celgene: Consultancy, Research Funding; Millennium: The Takeda Oncology Company: Research Funding; Novartis: Research Funding; Genzyme: Research Funding. Dispenzieri:Celgene, Millenium, Jansenn, Pfizer: Research Funding. Bergsagel:Onyx: Consultancy. Lacy:Celgene Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal