Abstract
The Janus-kinase (JAK) pathway plays a critical role in normal hematopoiesis. In two phase 3 studies, the JAK1/JAK2 inhibitor ruxolitinib (RUX) demonstrated clinical benefit over placebo (COMFORT-I) or best available therapy (COMFORT-II) in patients with intermediate-2 or high-risk myelofibrosis (MF). Anemia and thrombocytopenia, the most common adverse events associated with RUX treatment, were dose-dependent and expected based on RUX's mechanism of action. These events were successfully managed and few RUX-treated patients discontinued for anemia or thrombocytopenia (<1% for each event). The ability to identify patients who would most likely benefit from a dose adjustment may contribute to improved patient management. Therefore, an analysis was conducted in patients who received RUX in COMFORT-I to identify patient characteristics that could help select patients who would potentially benefit from closer monitoring and dose titration in the early period after initiation of therapy.
In COMFORT-I, starting doses in the patients randomized to RUX were 15 mg twice daily (BID) for patients with baseline platelet counts of 100–200 ×109/L (n=55) and 20 mg BID for those with platelet counts >200 ×109/L (n=100). Dose reductions were required for protocol-defined decreases in platelet counts; there was no protocol-mandated dose change for anemia. The time course of dose adjustments and the most common titrated doses by starting dose were evaluated. Logistic regression analyses with baseline hemoglobin (Hgb) level, platelet count, IPSS risk category, sex, and MF type as model effects were conducted to determine predictors of: 1) titration to a RUX dose of ≤10 mg BID within 8 weeks of starting therapy (early dose titration event); or 2) development of grade ≥3 anemia or new need for RBC transfusions (≥2 units of packed RBCs) within 12 weeks of therapy initiation (anemia event). Only baseline platelet count and Hgb emerged as significant predictors (alpha=0.05). Receiver operating characteristics (ROC) analyses were also conducted for these two predictors to determine a clinically relevant cutoff of the underlying predictor variable with a positive predictive value (PPV), true positive rate (TPR), and false positive rate (FPR) obtained at all possible cutoffs and a general goal of obtaining a TPR ≥ 50%, an FPR ≤ 10%, and a high PPV.
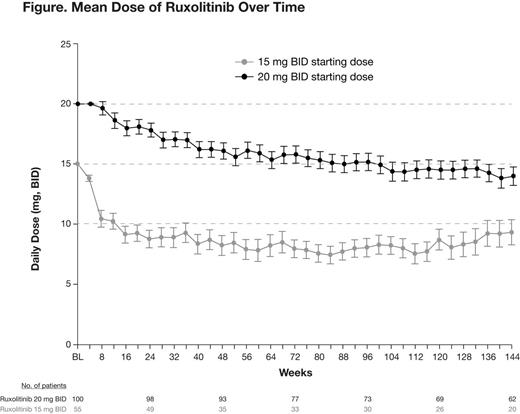
Approximately 70% of patients had a dose adjustment within the first 12 weeks of initiating RUX treatment. At week 24, 77% of patients with baseline platelet counts of 100–200 ×109/L and 39% of those with baseline platelet counts >200 ×109/L had titrated to a reduced RUX dose relative to their starting dose. With longer-term follow-up (median 149 weeks), the mean dose over time in patients who continued on study was ∼10 mg BID for patients starting at 15 mg BID and ∼15 mg BID for those starting at 20 mg BID (Figure).
ROC analyses identified that a baseline platelet count range of less than 150–175 x109/L may predict which patients would experience an early titration event, while a baseline Hgb level of less than 10 mg/dL may predict which patients would experience an anemia event (Table).
Predictive Characteristics of Cut-points for Platelet Count and Hgb
| Platelet Count/ Early Dose Titration Event . | Hgb/Anemia Event . | ||||||
|---|---|---|---|---|---|---|---|
| Cut-point (x109/L) | TPR | FPR | PPV | Cut-point (g/dL) | TPR | FPR | PPV |
| 125 | 18.5% | 3.3% | 80.0% | 8.5 | 13.6% | 0% | 100% |
| 150 | 43.1% | 5.6% | 84.8% | 9.0 | 22.7% | 3.1% | 83.3% |
| 175 | 52.3% | 7.8% | 82.9% | 9.5 | 31.8% | 4.6% | 82.4% |
| 200 | 60.0% | 16.7% | 72.2% | 10.0 | 45.5% | 10.8% | 74.1% |
| 225 | 69.2% | 21.1% | 70.3% | 10.5 | 61.4% | 18.5% | 69.2% |
| Platelet Count/ Early Dose Titration Event . | Hgb/Anemia Event . | ||||||
|---|---|---|---|---|---|---|---|
| Cut-point (x109/L) | TPR | FPR | PPV | Cut-point (g/dL) | TPR | FPR | PPV |
| 125 | 18.5% | 3.3% | 80.0% | 8.5 | 13.6% | 0% | 100% |
| 150 | 43.1% | 5.6% | 84.8% | 9.0 | 22.7% | 3.1% | 83.3% |
| 175 | 52.3% | 7.8% | 82.9% | 9.5 | 31.8% | 4.6% | 82.4% |
| 200 | 60.0% | 16.7% | 72.2% | 10.0 | 45.5% | 10.8% | 74.1% |
| 225 | 69.2% | 21.1% | 70.3% | 10.5 | 61.4% | 18.5% | 69.2% |
In COMFORT-I, most patients experienced a RUX dose adjustment in the first 12 weeks of treatment and stable doses were achieved with long-term therapy. Dose titration allowed most patients to maintain adequate platelet counts. After starting therapy at 15 or 20 mg BID, the majority of patients titrated to RUX doses of 10 mg BID or higher, which have been previously been shown to produce meaningful spleen volume reductions and symptom improvements (Mesa et al. 8th Annual Hematologic Malignancies Conference. Abstract 305; Verstovsek et al. ASH 2012. Abstract 800; Talpaz et al. ASH 2012. Abstract 176). A baseline platelet count of less than 150 ×109/L and baseline Hgb level of less than 10 g/dL may be clinically useful cutoffs for predicting which patients may benefit from closer monitoring shortly after treatment initiation of RUX and appropriate dose adjustments as needed.
Mesa:Lilly: Research Funding; Genentech: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; Incyte Corporation: Research Funding. Komrokji:Incyte Corporation: Membership on an entity’s Board of Directors or advisory committees. Sun:Incyte Corporation: Equity Ownership; Incyte Corporation: Employment. Sandor:Incyte Corporation: Equity Ownership; Incyte Corporation: Employment. Verstovsek:Incyte Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal