Abstract
In patients with imatinib-resistant chronic myeloid leukemia (CML) treated with a second generation tyrosine kinase inhibitor (2GTKI), the initial molecular response at 3 months (3m-IMR) has been shown to be predictive of long-term outcomes. However, there is no consensus regarding the best cutoff for the BCR-ABL1 transcript levels, with 1% and 10% being proposed in different studies. Also, additional prognostic factors such as baseline tyrosine kinase (TK) mutations and a prognostic scoring system (PSS) (Jabbour, 2011) have been proposed. In this study, we addressed those issues in an homogeneous group of imatinib-resistant CML patients treated in a single center for a median follow-up of 36 months (14-60).
134 patients with a diagnosis of CML treated either with dasatinib (N=64) or nilotinib (N=70) due to imatinib failure were included. Imatinib failure was defined as any of the following: no complete hematologic response at 3 months, no cytogenetic response at 6 months, no major cytogenetic response at 12 months, no complete cytogenetic response (CCR) at 18 months, or loss of hematologic or cytogenetic response at any time. Patients with the T315I mutation were excluded. Molecular analysis was performed in a properly standardized laboratory every 3 months from the beginning of the 2GTKI, and results are presented according to the international scale. The study's primary endpoint was the 3-year event-free survival (EFS) after 2GTKI. Secondary endpoints were the best response levels attained under 2GTKI (BMR). The factors analysed were the 3m-IMR, age, gender, Sokal and EUTOS scores at diagnosis, failure to achieve a complete cytogenetic response, TK mutations, PSS score and type of 2GTKI. A multivariate analysis with the factors significant at P<0.2 in the univariate analysis for EFS was also performed.
There were 74 males and 60 females, with a median age of 46 years (4-79). The Sokal score was available in 93 patients: it was low in 25 (27%), intermediate in 24 (26%) and high in 44 patients (47%). The EUTOS score was low in 61 and high in 32 patients. The best cytogenetic response to imatinib was complete in 32 patients, major in 18, minor in 12, and minimal or no response in 72 patients. The 62 patients who attained at least a minor cytogenetic response lost it before starting the 2GTKI. TK mutations were present in 33 patients (105 tested). The PSS score was low-risk in 59 (44%), intermediate in 61 (46%) and high-risk in 14 (10%) patients. The 3m-IMR was < 1% in 59 (44%) patients, 1% to <10% in 35 (26%) patients and ≥ 10% in 40 patients (30%). During the study period there were 16 deaths and 60 events. The adverse prognostic factors for the 3-year EFS in the univariate analysis were: 3m-IMR response 1% to <10% (HR of 6.15, <0.001), 3m-IMR ≥ 10% (HR 16.98 , P<0.001), failure to achieve a CCR (HR 4.44, P=0.001), presence of TK mutations (HR 1.76, P=0.044) and a high PSS score (HR 3.00, P<0.001). In the multivariate analysis, only the 3m-IMR remained predictive for EFS (HR 7.6 for 1% to<10% and HR 20 for ≥ 10%). Finally, the 3m-IMR was associated with the best response to 2GTKI: CCR of 100%, 49% and 15% (P<0.001); major molecular response of 88%, 31% and 10% (P<0.001), and molecular response 4.0 of 57.6%, 11.4% and 7.5% (P<0.001) for BCR-ABL1 transcript levels < 1%, 1% to < 10% and > 10%, respectively.
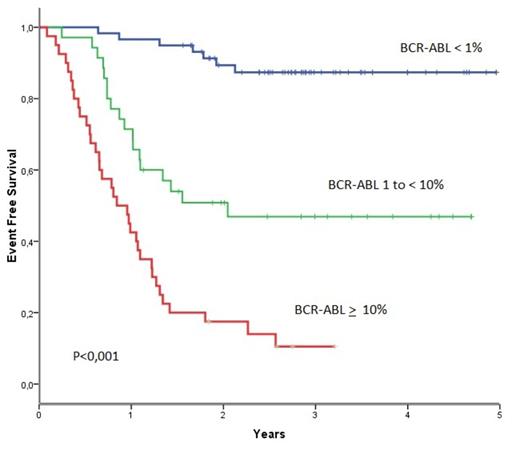
BCR-ABL1 transcript levels at 3 months identified a good-risk group (BCR-ABL1< 1%) with 3y-EFS of 87%, an intermediate-risk group (BCR-ABL1 1% to <10%) with 3y-EFS of 47% and a poor-risk group (BCR-ABL1 ≥ 10%) with 3y-EFS of 11% (P<0.001) (figure1). The present results confirm, in an independent series, that the BCR-ABL1 transcript level at 3 months is the most relevant surrogate for response and long-term outcome following 2GTKI after imatinib-failure in CML patients.
BCR-ABL1 transcripts at 3 months identify 3 risk groups : BCR-ABL1< 1% with 3y-EFS of 87%, BCR-ABL1 1% to<10% with 3y-EFS of 47% and BCR-ABL1 ≥ 10% with 3y-EFS of 11% (P<0.001)
BCR-ABL1 transcripts at 3 months identify 3 risk groups : BCR-ABL1< 1% with 3y-EFS of 87%, BCR-ABL1 1% to<10% with 3y-EFS of 47% and BCR-ABL1 ≥ 10% with 3y-EFS of 11% (P<0.001)
Boquimpani:BMS: Consultancy; Novartis: Consultancy, Research Funding. Lobo:Novartis: Research Funding. Spector:Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal