Abstract
In humans and other mammals, erythroid precursors in the bone marrow are the main consumers of iron. The availability of iron for erythropoiesis is controlled by hepcidin-induced endocytosis and degradation of ferroportin, the iron exporter which delivers iron to plasma from absorptive enterocytes and erythrocyte-recycling macrophages. In humans, within less than a day after hemorrhage or the administration of erythropoietin, duodenal iron absorption is increased by a mechanism which presumably evolved to provide for the iron requirement of increased erythropoiesis. Increased iron availability appears to be mediated by the suppression of the hormone hepcidin, thereby increasing ferroportin and delivering more iron to plasma. Increased erythropoietic activity is known to suppress hepcidin, but the molecular mechanism is not understood despite extensive investigation. We report that bleeding or administration of erythropoietin leads to the release of an erythroid factor made by erythroblasts which acts on hepatocytes to suppress hepcidin.
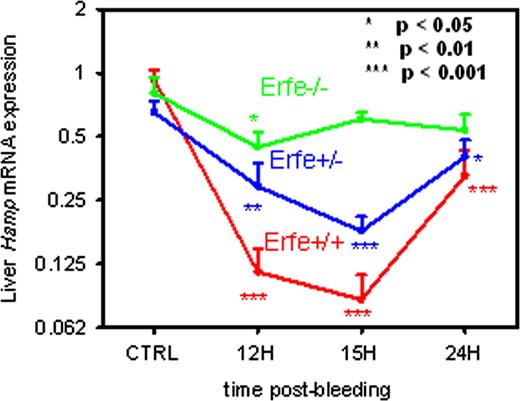
We examined the mouse hepcidin mRNA response to hemorrhage in wild-type mice or mice lacking hemojuvelin or TfR2, two of the critical mediators of hepcidin synthesis. Wild-type, hemojuvelin and iron-depleted TfR2 mutant mice all responded to hemorrhage by similar suppression of hepcidin mRNA within 9-15h, indicating that hemojuvelin and TfR2 are not essential for this response. We therefore initiated an unbiased search for potential suppressors of hepcidin by examining the time course of bone marrow response to hemorrhage (500 μl) using gene chip-based expression profiling. We identified less than a dozen erythroid-specific transcripts that change prior to the suppression of hepcidin mRNA. Searching for secreted proteins, we focused on a previously unidentified transcript that is highly induced prior to hepcidin suppression, and provisionally named it “erythroferrone” (Erfe). Erfe mRNA expression was greatly increased in the bone marrow and the spleen 4h after phlebotomy or EPO stimulation, preceding hepcidin suppression. Erfe-deficient mice did not suppress hepcidin mRNA after phlebotomy (Figure) or EPO injection and recovered more slowly from phlebotomy-induced anemia than their wild-type counterparts. We did not observe any significant defects in their baseline erythropoiesis or the composition or maturation of erythroid precursors suggesting that Erfe exerts its effect specifically on hepcidin for the regulation of iron availability. Hepcidin expression was reduced by injection of recombinant Erfe (2 μg/g) in wild-type mice. Moreover, treatment of mouse primary hepatocytes with supernatants of HEK293T cells overexpressing Erfe led to a significant decrease in hepcidin expression suggesting that Erfe can act directly on the liver to suppress hepcidin. Importantly, we also found that Erfe mRNA is greatly increased in the marrow and spleen of the mouse model of β-thalassemia Hbbth3/+ compared to wild-type controls.
Erythroferrone may be the long-sought erythroid factor repressing hepcidin during increased erythropoietic activity, and may contribute to the pathogenesis of iron-loading anemias including β-thalassemia.
Nemeth:Intrinsic LifeSciences: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees. Ganz:Intrinsic LifeSciences: Equity Ownership, Membership on an entity’s Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal